The COVID-19 pandemic continues to disrupt and end lives around the world, and public health officials worldwide have recognized vaccines as the critical tools required for controlling the COVID-19 death toll and achieving a return to normal life. Several vaccines against COVID-19 are already in use, but the limited supplies of these vaccines and the possibility of safety and efficacy issues of the existing vaccines mean that it is important for scientists to develop more (and even better) vaccines. In fact, as of February 2021, 69 different vaccines are in various phases of clinical development.
One type of vaccine that could prove quite useful is the inactivated vaccine, which contains an inactivated form of the virus. The inactivated virus cannot harm the recipient, but it still serves to trigger an immune response that causes the recipient’s immune system to produce antibodies that can later fight off the real virus if need be. Inactivated vaccines have been in use for decades and have several advantages, including a well-documented safety record, well-developed manufacturing processes, and the capacity to present multiple viral proteins for recognition by the immune system. So far, Chinese authorities have issued conditional approval to three different inactivated vaccines for use in controlling the COVID-19 pandemic.
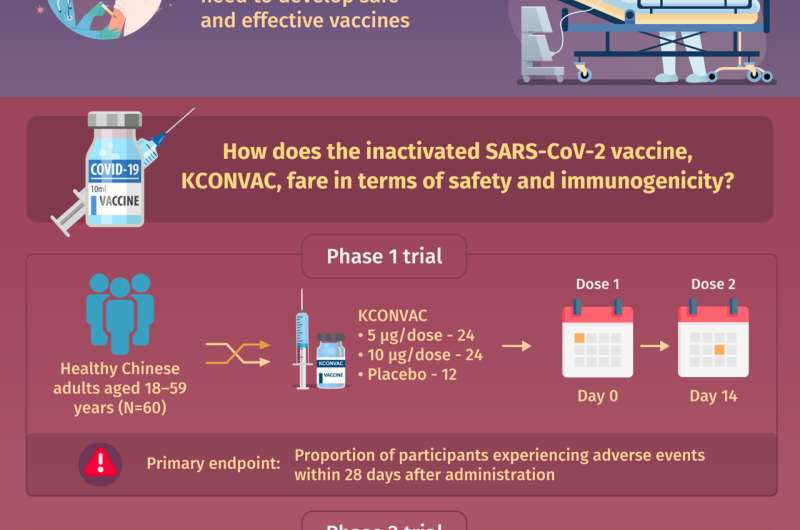
One inactivated vaccine currently in development is the KCONVAC vaccine developed by the Shenzhen Kangtai Biological Products Company and the Beijing Minhai Biotechnology Company. The KCONVAC vaccine is evaluated in ongoing phase 1 and phase 2 clinical trials, which aim to generate preliminary evidence of safety and preliminary evidence of efficacy, respectively. In a paper recently published in Chinese Medical Journal, a team of researchers led by Dr. Wen-Jie Tan of the Chinese Center for Disease Control and Prevention, Dr. Wei-Jin Huang of the China National Institutes for Food and Drug Control, and Dr. Feng-Cai Zhu of the Jiangsu Provincial Center for Disease Control and Prevention explore the existing clinical evidence for KCONVAC’s safety and ability to generate an immune response in healthy adults.
The phase 1 trial included 60 healthy Chinese adults, and the phase 2 trial included 500 healthy Chinese adults. In each trial, the participants were randomly assigned to groups that received 5 micrograms of the vaccine, 10 micrograms of the vaccine, or a placebo injection. The phase 2 trial participants received two separate injections administered either two or four weeks apart. Importantly, each trial was double-blind, meaning that neither the participants nor the clinical personnel they interacted with knew which group a participant had been assigned to. This “double-blinding,” a common procedure in clinical trials, served to increase the likelihood that any treatment effects would be due to the treatment administered rather than the participants’ expectations about what they would experience. “From the phase 1 and 2 trials, we wanted to know how many participants would experience adverse events within 28 days of the injections, and how effective the vaccine would be at inducing the production of antibodies against the novel coronavirus, respectively,” reports Dr. Tan.
In the phase 1 trial, participants who received the vaccine were no more likely to experience adverse events than participants who received the placebo injections were. The same pattern emerged in the phase 2 trial, and no severe vaccine-related adverse events occurred. In the phase 2 trial, KCONVAC successfully induced antibody production, and antibody production was stronger in participants who received their injections four weeks apart than in those who received their injections two weeks apart.
Dr. Huang says, “These findings show that KCONVAC, both at a 5-microgram dose and a 10-microgram dose, is well tolerated and able to induce robust immune responses in adults and support the testing 5-microgram vaccine doses spaced four weeks apart in an upcoming phase 3 trial.”
Source: Read Full Article
