In a recent study published in the journal Molecular Therapy, researchers identified Bifonazole, an imidazole-based antifungal agent, to have potent activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using high-throughput screening of small molecules.
SARS-CoV-2 infects host cells through interactions of the spike (S) protein receptor-binding domain (RBD) and angiotensin-converting enzyme 2 (ACE2) receptors of the host. Therefore, the S RBD-ACE2 binding site is a potential target for the development of anti-SARS-CoV-2 therapies.
 Study: Identification of FDA-approved Bifonazole as SARS-CoV-2 blocking agent following a bioreporter drug screen. Image Credit: NIAID
Study: Identification of FDA-approved Bifonazole as SARS-CoV-2 blocking agent following a bioreporter drug screen. Image Credit: NIAID
About the study
In the present study, researchers explored the use of small molecules in anti-SARS-CoV-2 therapeutics by identifying and comparing the efficacy of small molecules that blocked S RBD-ACE2 interactions.
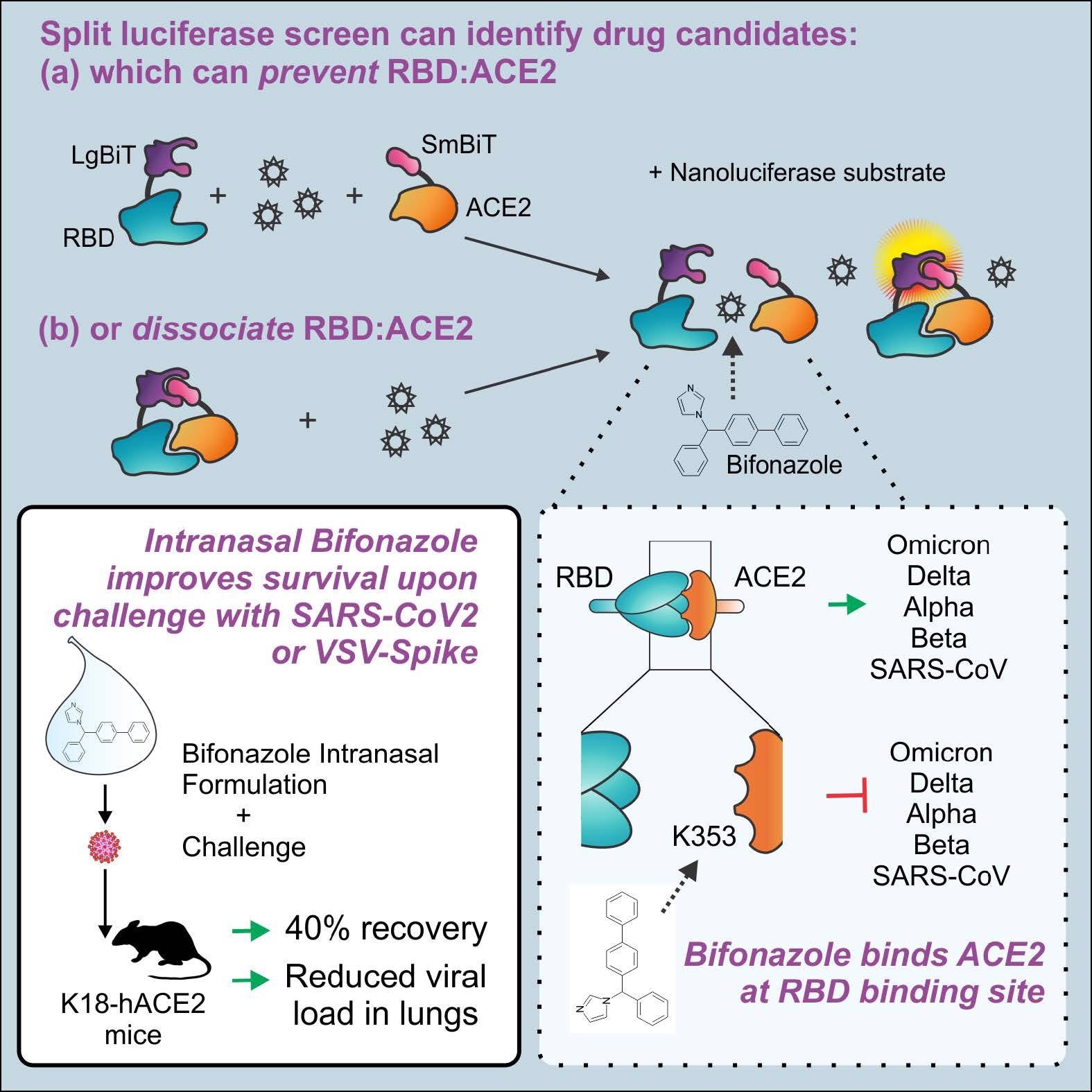
The team used a split-luciferase assay for screening small molecules that inhibit S RBD-ACE2 binding. The assay was based on the NanoLuc Binary Technology (NanoBiT), in which the nanoluciferase bioreporter was dissected into two parts, i.e., the Small BiT (SmBiT) and Large BiT (LgBiT), which exhibited robust conformational stability and thereby created an ideal split reporter for investigating the S RBD-ACE2 interactions. In the assay, the S RBD-ACE2 binding gave strong and stable luminescent nano-luciferase signals upon adding the coelenterazine (CTZ) substrate.
 © 2022 The American Society of Gene and Cell Therapy
© 2022 The American Society of Gene and Cell Therapy
Human embryonic kidney 293 (HEK 293) cells were transfected to express the LgBiT-RBD and SmBiT-ACE2 proteins. Further, LgBiT-RBD interactions with anti-S RBD antibodies or SmBiT-ACE2 interactions with soluble ACE2 (sACE2) demonstrated competitive inhibition and impaired the luminescence signals. A total of 1,200 Food and Drug Administration (FDA)-approved drug compounds of the Prestwick library were screened using two methods A and B.
In Method A, the compound library was added to the LgBiT-RBD/SmBiT-ACE2 complex for identifying compounds that dissociated the complex. In Method B, the library was added to the LgBiT-RBD complex followed by adding SmBiT-ACE2 to identify compounds that inhibited the S RBD-ACE2 interactions. Hits obtained with bioreporter luminescence ≤ 15 % were eliminated from the analysis and compounds with the top 45 hits were further analyzed.
The efficacy of the compounds was assessed in ACE2- and transmembrane serine protease 2 (TMPRSS2)-expressing VeroE6 cells infected with SARS-CoV-2 S pseudotyped vesicular-stomatitis virus (VSV) expressing green fluorescent protein (VSV-GFP-S) or wild type (wt)VSV-GFP. Subsequently, immunofluorescence imaging and cell viability assessments were performed. In addition, the efficacy of Bifonazole in K18-ACE2 mice was assessed.
Graphs denoting % GFP foci for wtVSV and VSV-S across all drug concentrations were plotted to assess the specificity of the compounds. In addition, in silico molecular docking analysis and bimolecular fluorescence complementation (BiFC) assays were performed and the SARS-CoV-2 messenger ribonucleic acid (mRNA) levels were assessed.
Results
Among the 1,200 compounds screened, Bifonazole (Prestw-1241) showed the most potent competitive inhibition of SARS-CoV-2 S RBD-ACE2 interactions using both methods, with a decrease of 46% and 51% in the bioreporter signals using methods A and B, respectively. In silico molecular docking analysis showed that Bifonazole was bound to the N-terminal small lobe of ACE2 around the K353 residue, which resulted in the prevention of RBD-ACE2 binding.
Bifonazole significantly reduced the pseudotyped VSV-S-GFP infected cells [half-maximal inhibitory concentration (IC50) = 30 to 40 µM, without toxicity up to 100 µM] and wtVSV-GFP infected cells (IC50 = 60 to 70 µM). When administered intranasally, Bifonazole led to a 40% reduction in lethality in VSV S-challenged K18-ACE2 mice with similar benefits post-challenge with live SARS-CoV-2.
The specificity graphs of Bifonazole showed an upward trend from 20 to 67 μM, indicative of the compound’s potential for VSV-S inhibition. Bifonazole also reduced SARS-CoV-2 mRNA levels, reduced viral burden in the median tissue culture infectious dose (TCID50) assay, and reduced immunofluorescence staining for SARS-CoV-2 S. Cytotoxicity assays confirmed no impact of Bifonazole on cell viability, as observed in the VeroE6-hTMPRSS2 cells at the doses tested. Moreover, Bifonazole demonstrated significantly reduced the luminescence of mutant RBD bioreporters, indicative of substantial inhibition of SARS-CoV-2 variants (especially Omicron) that bind with ACE2 by Bifonzaole.
The BiFC assay showed a dose-dependent decrease in the fusion of TMPRSS2/ACE2-expressing cells and S-expressing human embryonic kidney (HEK293T) cells on Bifonazole treatment. Similar effects were observed on using S of SARS-CoV with 45% and 22% inhibition of the SARS-CoV S RBD-ACE2 interactions using methods A and B, respectively. This indicated that Bifonazole also possessed antiviral activity against SARS-CoV.
Among the other compounds, nicardipine and ethynylestradiol significantly reduced bioreporter signals with comparable bioreporter impairment between Bifonazole and nicardipine (IC50 = 1.9 µM), cisapride (IC50 = 16.5 µM), and ethynylestradiol (IC50 = 2.6 µM). Nicardipine and ethynylestradiol inhibited wtVSV-GFP (IC50 = 15 to 20 µM) more efficiently than VSV-S-GFP (IC50 = 25 to 70 µM). Cisapride did not affect VSV infection and was toxic at >20 µM doses. The specificity curves for nicardipine and ethynylestradiol showed a downward trend, indicating enhanced wtVSV inhibition and lower S specificity.
In the drug screening, other imidazole antifungals (econazole, ketoconazole, clotrimazole, oxiconazole nitrate) and an imidazole anti-inflammatory agent, tribenoside, blocked the S RBD-ACE2 interactions. Econazole (IC50 = 2.3 µM) and ketoconazole (IC50 = 14.5 µM) showed bioreporter impairment and upward trends in the specificity curves, albeit at high concentrations of 60 to 80 μM, respectively.
Conclusion
Overall, the study findings highlighted the promising potential of Bifonazole as an anti-SARS-CoV-2 therapeutic agent with high potency and specificity for SARS-CoV-2 inhibition by competitively binding to ACE2 and subsequently blocking the S RBD-ACE2 interactions.
- Taha Z, Arulanandam R, Maznyi G, Godbout E, Carter-Timofte ME, Kurmasheva N, Reinert LS, Chen A, Crupi MJF, Boulton S, Laroche G, Phan A, Rezaei R, Alluqmani N, Jirovec A, Acal A, Brown EEF, Singaravelu R, Petryk J, Idorn M, Potts KG, Todesco H, John C, Mahoney DJ, Ilkow CS, Giguère P, Alain T, Côté M, Paludan SR, Olagnier D, Bell JC, Azad T, Diallo J-S, Identification of FDA-approved Bifonazole as SARS-CoV-2 blocking agent following a bioreporter drug screen, Molecular Therapy (2022), DOI: https://doi.org/10.1016/j.ymthe.2022.04.025, https://linkinghub.elsevier.com/retrieve/pii/S1525001622002969
Posted in: Drug Discovery & Pharmaceuticals | Medical Research News | Biochemistry | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antifungal, Anti-Inflammatory, Assay, BiFC, Cell, Compound, Coronavirus, Coronavirus Disease COVID-19, Cytotoxicity, Efficacy, Enzyme, Fluorescence, Fluorescent Protein, Food, Gene, High-throughput screening, Imaging, Kidney, Luciferase, Omicron, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Serine, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Stomatitis, Syndrome, Therapeutics, Tissue Culture, Virus

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article
