Organoids are three-dimensional cell cultures from embryonic stem cells or induced pluripotent stem cells that model important features of whole organs.
 Credit: nobeastsofierce/Shutterstock.com
Credit: nobeastsofierce/Shutterstock.com
Undifferentiated stem cells are able to reaggregate and replicate the architecture of the original organ. Hence, they are used to produce organoids for biomedical research.
Current applications of organoids include utilization in the study of human development and disease. Developments in personalized and regenerative medicine are also being aided by organoid systems.
Organoids in biomedical research
Since organoids are derived from human stem cells or induced pluripotent stem cells, they provide the genetic and physiological similarity required for the enhanced modeling of human development and disease, in comparison to current animal models.
Organoids are currently being employed in biomedical research to:
- Examine organ development and tissue morphogenesis.
- Model diseases.
- Test drug sensitivity and toxicity.
- Potentially form complex tissues for transplantation.
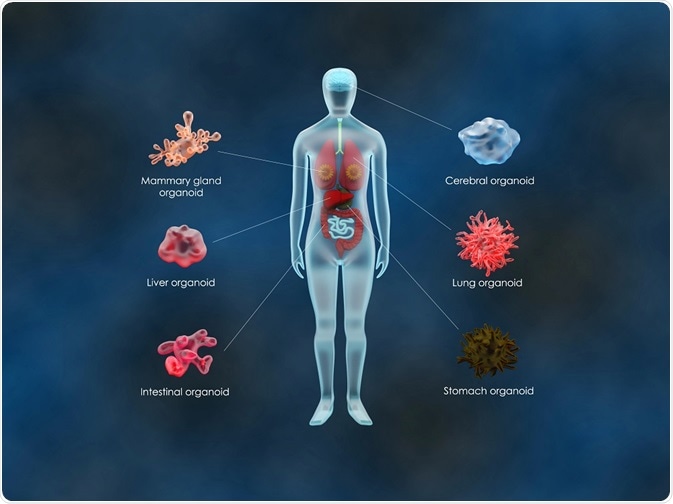
Organoids of the intestine, liver, kidney and brain have all been successfully developed with the potential for future organoid systems that recapitulate organs, such as the heart and lungs.
The modeling of human development and disease is enhanced through the application of organoids because they only require a small number of cells in order to form a large range of tissue types.
Experiments can be easily controlled through pure cultures of specific tissue types whilst advances in bioengineering mean that exact modeling can be produced, reflecting complex interactions.
Organoids and regenerative medicine
Organoids may be applied to the field of regenerative medicine. By combining organoid systems with biofabrication strategies, complex tissue or organ functions can be replicated. Current advances in developing intestinal organoids are providing the potential for organoid-based therapeutic treatments for patients with short bowel syndrome and human inflammatory bowel disease.
The aim is to produce a standardized procedure to restore intestinal epithelial function. Organoids may be employed as a source of intestinal regenerative medicine by developing the organoid within a complex three-dimensional scaffold to supply functional tissues. Intestinal organoids may also be transplanted back into the body to undergo functional maturation.
Studies have already successfully grafted human colonic stem cells to mouse colons providing evidence that intestinal epithelial organoid cells may successfully be used in a transplant-based therapy.
 Credit: Meletios Verras/Shutterstock.com
Credit: Meletios Verras/Shutterstock.com
Organoids and personalized medicine
Personalized medicine promises to provide patients with the most suitable treatment that is specific to them. Organoid systems are an important tool in developing personalized medicine because they are derived from a single patient biopsy meaning that the culture will display genetic similarity.
Tests of drug efficacy can therefore be performed on organoid systems rather than the lengthy trial and error testing of treatments prescribed to the patient.
The methodology has already proven successful in identifying individual treatment outcomes for cystic fibrosis. The mutated cystic fibrosis transmembrane conductance regulator (CFTR) affects the production of the CFTR protein which causes cystic fibrosis.
CFTR modulators help modify the defective protein but have been found to produce varied responses in patients. Intestinal organoids have been successfully used to measure the individual efficacy of CFTR modulators.
The results were found to reflect in vivo responses to the drug meaning that organoid systems can provide a more efficient way of predicting patient response to cystic fibrosis treatment.
Organoids and cancer research
Organoid systems are currently being used for modeling cancer development and treatments. Cancer research has been limited due to the lack of in vitro models that can accurately replicate the physiology of the original tumor.
In 2017, organoids of primary liver cancers were propagated preserving the physiological architecture and gene expression of the original tumor.
The results of in vivo transplantation in mice found that the long term in vitro expansion of the cancer-based organoids still preserved the parent tumor histology, by the secondary tissues derived from the grafted tumors exhibiting similar chromosome counts and identical morphology to the parental line.
Moreover, the cancer-based organoids were utilized in a successful array for drug sensitivity testing. The results mean that organoid systems which maintain the histology and gene expression of the original tumor can be employed in predicting drug sensitivity and specific patience response to cancer treatments in future.
Sources:
- Xinaris, C. et al. 2015. Organoid Models and Applications in Biomedical Research, Experimental Nephrology and Genetics: Review, 130, pp. 191-199.
- Broutier, L. et al. 2017. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening, Nature Medicine, 23, pp. 1424-1435.
- Nakamura, T. & Sato, T. 2018. Advancing Intestinal Organoid Technology toward Regenerative Medicine, Cellular and Molecular Gastroenterology, 5, pp. 51-60.
- Noordhoeck, J. et al. 2016. Intestinal organoids and personalized medicine in cystic fibrosis: a successful patient-oriented research collaboration, Current Opinion in Pulmonary Medicine, 22, pp. 610-616.
Further Reading
- All Cell Content
- Structure and Function of the Cell Nucleus
- What Are Organelles?
- Cilia and Flagella in Eukaryotes
- Mitosis vs Meiosis
Last Updated: Feb 26, 2019

Written by
Shelley Farrar Stoakes
Shelley has a Master's degree in Human Evolution from the University of Liverpool and is currently working on her Ph.D, researching comparative primate and human skeletal anatomy. She is passionate about science communication with a particular focus on reporting the latest science news and discoveries to a broad audience. Outside of her research and science writing, Shelley enjoys reading, discovering new bands in her home city and going on long dog walks.
Source: Read Full Article
