Prion diseases are incurable, deadly neurological disorders that can affect both humans and animals—including Creutzfeldt-Jakob Disease (CJD) in people, Bovine Spongiform Encephalopathy (also known as Mad Cow Disease) and Chronic Wasting Disease found in deer.
Scientists have spent decades working to better understand how prions cause these diseases. But new research from the Case Western Reserve University School of Medicine and the National Institutes of Health (NIH) Rocky Mountain Laboratories brings into focus how prions might be formed and how they can result in different disease outcomes.
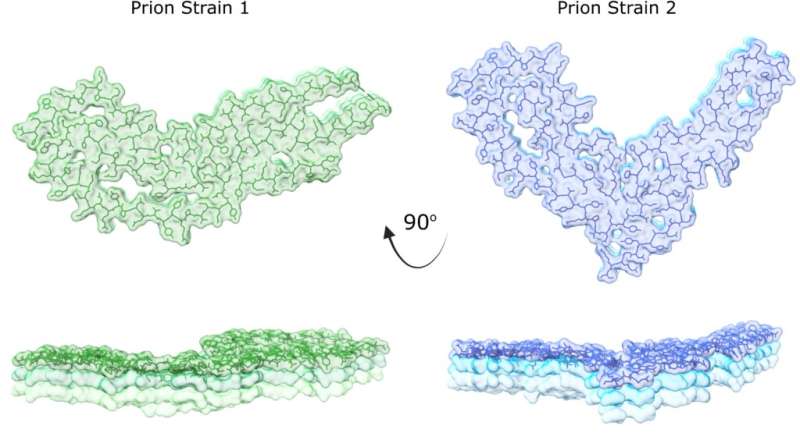
This collaborative research team discovered a new prion structure through revolutionary new imaging using specialized electron microscopes at the Cleveland Center for Structural and Membrane Biology Cryo-Electron Microscopy Core and the Rocky Mountain Laboratories. The new structure is of a second prion strain, providing an atomic-level view into how corrupted prion proteins self-convert and assemble.
“Prions are unusual pathogens. Different prions result in different disease lengths and symptoms in their host,” said Allison Kraus, assistant professor of pathology at the School of Medicine, who co-led the research with Byron Caughey at NIH. “This has long perplexed scientists because different diseases occur with corruption of the same protein from its original shape.”
The research was published this month in Nature Communications.
“Our study identifies the key similarities and differences in prions and how these features compare to other types of self-converting proteins,” Kraus said. “We can now begin to appreciate how those key similarities and differences shape disease outcomes of prion disease and compare the outcomes to other brain diseases.”
Prion disease occurs when prion proteins are refolded, causing a chain reaction where other prion proteins refold and accumulate in the brain, according to the NIH.
Kraus said the new prion imaging could play a key role towards understanding prion disease outcomes.
Source: Read Full Article
