The existential threat of COVID-19 has highlighted an acute need to develop working therapeutics against emerging health threats. One of the luxuries deep learning has afforded us is the ability to modify the landscape as it unfolds—so long as we can keep up with the viral threat, and access the right data.
As with all new medical maladies, oftentimes the data needs time to catch up, and the virus takes no time to slow down, posing a difficult challenge as it can quickly mutate and become resistant to existing drugs. This led scientists from MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) to ask: how can we identify the right synergistic drug combinations for the rapidly spreading SARS-CoV-2?
Typically, data scientists use deep learning to pick out drug combinations with large existing datasets for things like cancer and cardiovascular disease, but, understandably, they can’t be used for new illnesses with limited data.
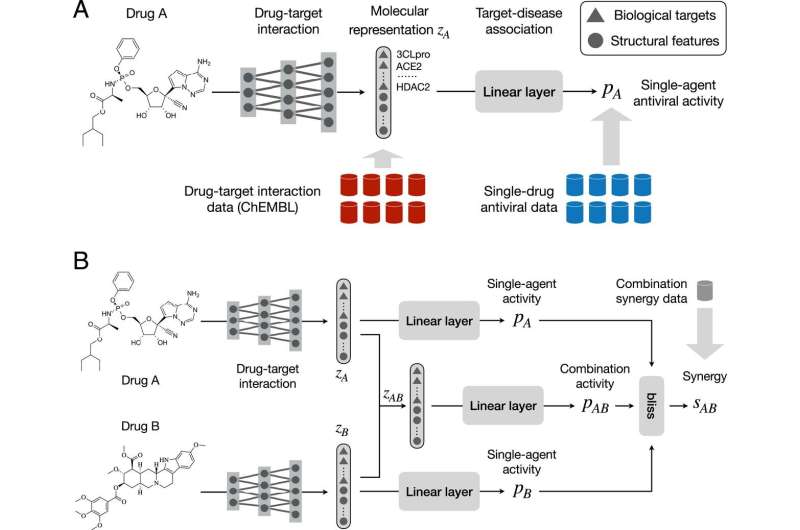
Without the necessary facts and figures, the team needed a new approach: a neural network that wears two hats. Since drug synergy often occurs through inhibition of biological targets, (like proteins or nucleic acids), the model jointly learns drug-target interaction and drug-drug synergy to mine new combinations. The drug-target predictor models the interaction between a drug and a set of known biological targets that are related to the chosen disease. The target-disease association predictor learns to understand a drug’s antiviral activity, which means determining the virus yield in infected tissue cultures. Together, they can predict the synergy of two drugs.
Two new drug combinations were found: remdesivir (currently approved by the FDA to treat COVID-19), and reserpine, as well as remdesivir and IQ-1S, which, in biological assays, proved powerful against the virus.
“By modeling interactions between drugs and biological targets, we can significantly decrease the dependence on combination synergy data,” says Wengong Jin, CSAIL Ph.D. and MIT Broad Institute postdoc, the lead author on a new paper about the research. “In contrast to previous approaches using drug-target interaction as fixed descriptors, our method learns to predict drug-target interaction from molecular structures. This is advantageous since a large proportion of compounds have incomplete drug-target interaction information.”
Using multiple medications to maximize potency, while also decreasing side effects, is practically ubiquitous for aforementioned cancer and cardiovascular disease, including a host of others such as tuberculosis, leprosy, malaria. Using specialized drug cocktails can, quite importantly, reduce the grave, sometimes public threat of resistance, (think methicillin-resistant Staphylococcus aureus known as “MRSA”) since many drug-resistant mutations are mutually exclusive. It’s much harder for a virus to develop two mutations at the same time, and then become resistant to two drugs in a combination therapy.
The model also isn’t limited to just SARS-CoV-2—it could also be used for the increasingly contagious delta variant. To extend it there, you’d only need additional drug combination synergy data for the mutation. The team also applied their approach to HIV and pancreatic cancer.
To further refine their biological modeling down the line, the team plans to incorporate additional information such as protein-protein interaction and gene regulatory networks.
Another direction for future work they’re exploring is something called “active learning.” Many drug combination models are biased toward certain chemical spaces due to their limited size, so there’s high uncertainty in predictions. Active learning helps guide the data collection process and improve accuracy in a wider chemical space.
Source: Read Full Article
