Acute myeloid leukemia, which affects blood and bone marrow cells, is a particularly dangerous form of cancer. More than half of patients under the age of 60 die. This proportion rises to 85% for patients over 60. A team from the University of Geneva (UNIGE), Switzerland, and from Inserm, in France, have identified a previously unknown mechanism that could lead to the development of new therapies. The selective activation of AMPK, a key enzyme in the energy balance of tumor cells, would indeed lead to their death by triggering the cells stress response. Moreover, the scientists have successfully exploited this energy gap in an animal model of the disease: a combination of two drugs—one of which is already on the market—has indeed shown promise. However, their effectiveness has yet to be confirmed on leukemia stem cells, which have the ability to escape many treatments to restart tumor growth. These results can be found in the journal Cell Reports.
Jérôme Tamburini, an associate professor in the Department of Medicine and in the Translational Research Centre in Onco-Haematology (CRTOH) of UNIGE Faculty of Medicine and at the Swiss Cancer Center Léman (SCCL) and a professor at Université de Paris, is working on the energetic mechanisms of tumor cells in acute myeloid leukemia. A cell signaling pathway called AMPK is of particular interest to him. “AMPK is the main detector of the cells energy level,” explains Jérôme Tamburini. “This pathway is activated when energy is lacking and initiates the degradation of certain nutrients to produce the necessary energy—a process called catabolism. As without energy, no cell can survive, could it be possible to selectively manipulate this mechanism in tumor cells to cause their destruction, while preserving healthy cells?”
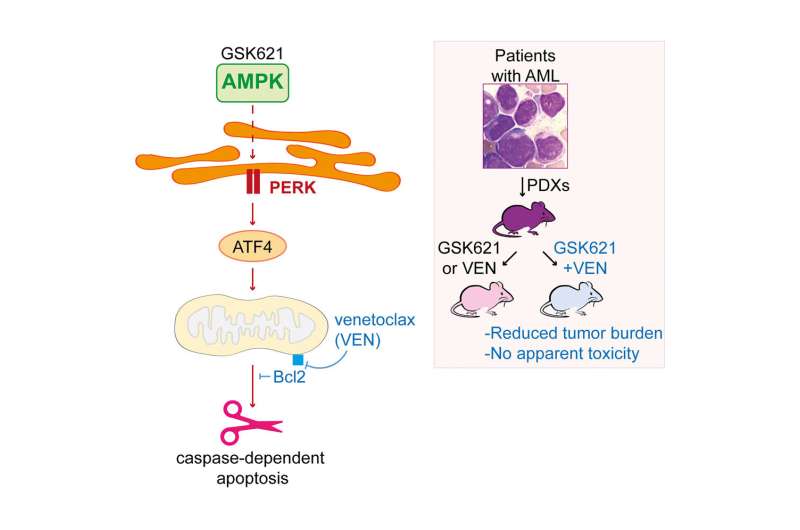
In 2015, Jérôme Tamburini and his colleagues at Inserm in Paris participated in the development with the GlaxoSmithKline (GSK) laboratory of a pharmacological component—GSK621—which proved to be an excellent activator of AMPK in vitro. “After this initial proof of principle, we had to decipher the biochemical mechanisms at work in order to understand them in detail, and in particular which cellular pathways did GSK621 activate in leukemia cells, the first step in hoping to exploit this phenomenon for therapeutic purposes,” explains Jérôme Tamburini.
An effective combination of two drugs
The first step was to perform a gene expression analysis of human tumor cells, which identified an enzyme, PERK, particularly activated in response to the presence of GSK621. This is a key element in the stress response of the endoplasmic reticulum, an intracellular structure specialized in the metabolism of proteins and lipids. “The activation of AMPK thus triggers the activation of PERK, followed by a chain of reactions leading to apoptosis, the programmed death of the cell,” explains Jérôme Tamburini. “In addition, the activation of AMPK by GSK621 sensitizes the cells to the effects of another pharmacological drug, the venetoclax, which is now widely used to treat acute myeloid leukemia, although with limited effectiveness when used alone.”
The scientists then combined the two drugs in mice carrying human tumor cells, and found that this combination controlled tumor development much more effectively than in monotherapy. While GSK621 was not designed to be a drug, other products are currently in clinical trials to combat metabolic diseases, which activate the AMPK pathway. “Understanding the mechanism involved has brought to light potential therapeutic targets that were previously unknown,” explains Jérôme Tamburini. “We will now be able to review all the drugs known to have an effect on these pathways and determine which combinations would be the most effective.”
What about leukemic stem cells?
Source: Read Full Article
