Transparent zebrafish skin was used to explore how pre-cancerous cells behave, enhancing our understanding of how cancers progress.
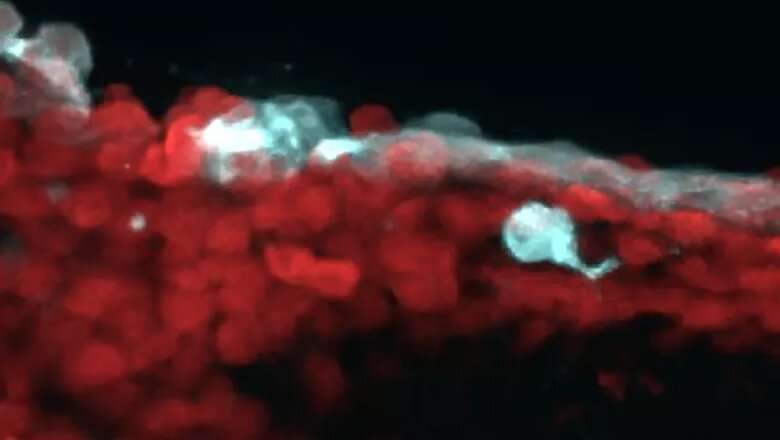
The study, conducted by the Rosenblatt Lab and today published by Nature Communications, made use of the transparent skin of developing zebrafish embryos to model the behaviors of transformed, pre-cancerous cells.
The researchers enforced the expression of fluorescent proteins within transformed cells in their zebrafish model to observe them under the microscope. They discovered that, very early on, these transformed cells hijack a mechanism that normally promotes cell death to instead allow their invasion into the underlying tissue. This mechanism is called basal cell extrusion.
Understanding this new mechanism has highlighted how cells can invade before full-blown tumors even form, and at sites independent of primary masses—a theme rapidly emerging in aggressive tumors like pancreatic cancer. It also provides a new understanding of how they can change their fate and transform into new cell types that we might be missing when screening for cancer.
Most solid cancers form from a tissue called epithelia, which serves as a barrier or “skin” that coats our internal organs. This process mechanically strips off the cells’ epithelial identity, enhancing their migratory ability very early on in cancer progression.
The research originated several years ago when the lab studied transformed cells in petri dish cultures and observed that cells are extruded not only apically (i.e. above the monolayer) but also basally (i.e. below the monolayer). They hypothesized then that this process may drive cell invasion into tissue. They chose embryonic zebrafish skin to model simple epithelia where cancers arise as they are completely transparent, allowing researchers to follow invasion live.
If their findings hold true in humans, they could shed light on how cells invade to metastasize to other sites. The lab is now imaging the same process in mice tissue and has found that cells appear to invade similarly, suggesting this could be a conserved mechanism in humans as well. Since metastasis is the main reason patients succumb to cancer, understanding how it is initiated will be key to preventing it.
The researchers are also finding the mechanical pressure that cells experience after they invade (due to obstacles they encounter in the tissue) can impact their fates, turning them into more fibrotic, more difficult to treat cancer cell types. As a result they are investigating if other cancer-causing mutations also disrupt the extrusion pathway, driving invasion similarly.
Source: Read Full Article
