Skip to
- What is Pluripotency?
- How is Pluripotency maintained?
- Other Factors Involved in the Maintenance of Pluripotency
- Induction of pluripotent cells from somatic (differentiated cells)
Stem cells retain the ability to divide throughout life and give rise to progenitors of specialized cells to replace those that have died or are lost from a tissue. These features are referred to as self-renewal and differentiative division, respectively. The combination of these two types of cell division gives long term reconstitution of stem cells, subsequently providing a lifetime supply of these cells and their progeny. This article focuses on embryonic stem cells (ESCs) as an example of pluripotent stem cells.
What is Pluripotency?
Not all stem cells possess an equal potential to produce specialized cell types. The sum potential of a cell to give different specialized cell types is called ‘potency’. ESCs possess pluripotency. Pluripotency refers to the ability of a cell to generate the three major embryonic germ layers: the endoderm, ectoderm and mesoderm. All three lineages give rise to any cell type in the body.
How is Pluripotency maintained?
There are numerous factors thought to influence the maintenance of the undifferentiated state. These can be divided into intrinsic factors and extrinsic factors:
- Intrinsic factors relate to the processes that occur within the cell.
- Extrinsic factors are those which influence the cell from external sources.
The most important intrinsic factors are transcription factors. Of these, there are three master regulators: the Octamer-binding transcription factor (OCT4), Sex-determining region Y-box 2 (SOX2) and Nanog. All three act together to prevent differentiation of one of two populations of cells that are formed by the 8-cell stage embryo. The inner population forms the inner cell mass (ICM), while the outer population becomes the trophectoderm (TE). The cells of the ICM house the pluripotent ESCs.
OCT4 helps establish the ICM in a mutually antagonistic relationship with another factor called Cdx-2. This is illustrated here:
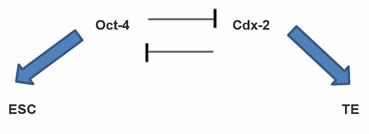
Another interacting pair of transcription factors are OCT4 and Nanog. They sequentially prevent the differentiation of the ICM — first to trophectoderm (via OCT4) then to primitive endoderm (Nanog).
OCT4 can also affect gene expression by forming a complex with SOX2 in the cell nucleus. This is important both in maintaining pluripotency and determining lineage specialization. This function can be seen in mice in which SOX2 has been knocked out. SOX2 knock-out mice suffer peri-implantation death due to the inability of cells to form the epiblast, derived from the ICM, which give rise to the three lineages (mesoderm, endoderm and ectoderm).
The amount of OCT4 and Nanog must be controlled; overexpression of OCT4 causes ICM cells to differentiate into primitive endoderm and mesoderm. Nanog, which also maintains pluripotency, can similarly cause differentiation of the ICM into primitive endoderm if it is under-expressed.
Other Factors Involved in the Maintenance of Pluripotency
There are several extrinsic factors that affect pluripotency. The most notable are:
- LIF – This cytokine maintains ES cells through the activation of the JAK/STAT signalling pathway, which increases the transcription of genes involved in self-renewal. However, LIF can also activate the ERK pathway which stimulates differentiation. A balance between the two is therefore necessary to maintain ES cells and control differentiation.
- Transforming Growth Factor β (TGF-β ) – Two groups of the TGFβ superfamily are involved in the control of self-renewal and differentiation in human ES cells: bone morphogenetic protein (BMP) and nodal and activin signalling molecules.
- BMP – BMP proteins, specifically BMP-2 and -4, bind surface receptor heterodimers which cause up-regulation of Id (inhibitors of differentiation). This occurs via SMAD 1, 5 and 8 proteins that also reduce Oc=CT4 and Nanog signalling resulting in the differentiation of trophoblast.
- Activin and nodal signalling – these signalling molecules work in opposition to BMP and act via the Smad2/3 pathway to stimulate Nanog and inhibit human ESC cell differentiation – specifically neural differentiation.
- FGF2 and Wnt Signaling – These act synergistically with activin and nodal to maintain the pluripotency of human ES cells
There are several other intrinsic factors which also affect pluripotency. The most important is epigenetics, which are changes in gene expression that do not involve changes in the DNA sequence. Epigenetic changes include post-translational modification of the DNA by acetylation and methylation. Acetylation typically stimulates decompaction of chromatin which enables genes to be more accessible to transcription factors. This has the effect of enabling the transcription of genes in this region. Conversely, methylation encourages tight packing of chromatin, thereby rendering genes inaccessible to the transcriptional machinery. This has the effect of preventing the transcription of the genes in this region. Subsequently, the control of the transcriptional status of the chromatin affects the differentiation and specialization of the cell.
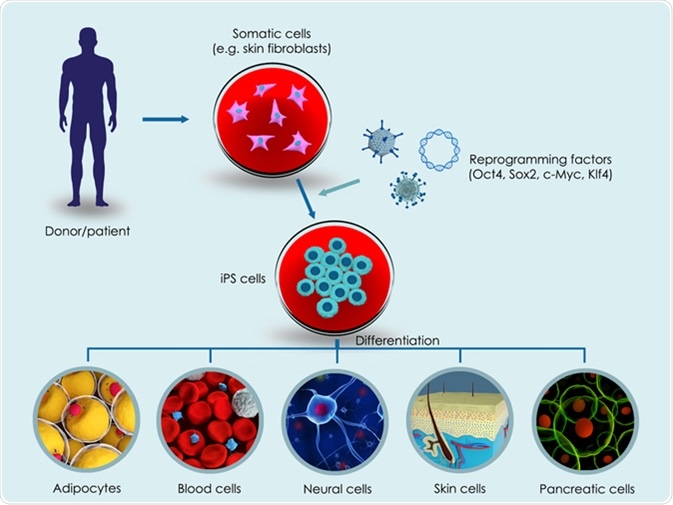
Induction of pluripotent cells from somatic (differentiated cells)
Historically, differentiation has been regarded as irreversible, with cellular reprogramming to return to the undifferentiated state thought to be impossible. However, the first example of reprogramming somatic cells to the pluripotent state in 1962 by Sir John Gurdon disproved this dogma. Gurdon produced a tadpole by combining a frog egg cell devoid of a nucleus (enucleated) with the nucleus from an intestinal epithelial somatic cell of a tadpole. This process was termed somatic cell nuclear transfer (SCNT). SCNT was implemented in the cloning of Dolly the sheep. This revealed that the somatic cell possessed the necessary information to generate a whole organism and the egg cell possessed factors capable of initiating cell reprogramming. In 2001 cell fusion was revealed as an alternative strategy of iPSC generation; a process involving fusion of somatic cells with ESCs. This strategy paved the way for the production of mouse ESCs cell lines in 1981, and then human ESCs in 1998.
The most recent advance in somatic reprogramming was provided in 2006 with the discovery of induced pluripotent stem cells (iPSCs) by the researcher Shinya Yamanaka. These cells are somatic (body) cells that have undergone reprogramming via the ectopic expression of a cocktail of transcription factors expressed in ESCs.
Yamanaka used a retrovirus to deliver four reprogramming transcription factors into a somatic mouse fibroblast. These factors were Oct 3/4 (Octamer-binding transcription factor-3/4), Sox2 (Sex-determining region Y)-box 2, Klf4 (Kruppel like factor-4), and c-Myc. In 2007, the same principle was applied to a somatic human fibroblast to produce human iPSCs (hiPSCs) using a different delivery system (vector) called a lentivirus and different reprogramming factors; Oct 3/4, Sox2, Nanog, and Lin 28.
This reprogramming causes cells to acquire the characteristic of an embryonic stem cell. iPSC generation bypasses may ethical concerns associated with ESCs. They avoid the ethical concerns surrounding embryo destruction; safety concerns resulting from recipient rejection of donor ESCs; and, the limited embryo availability. Subsequently, iPSCs provide a source of pluripotent stem cells without any associated ethical drawbacks.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4661882/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4550282/
- www.eurostemcell.org/ips-cells-and-reprogramming-turn-any-cell-body-stem-cell
- https://stemcells.nih.gov/info/Regenerative_Medicine/2006chapter10.htm
Further Reading
- All Pluripotency Content
Last Updated: May 21, 2019

Written by
Hannah Simmons
Hannah is a medical and life sciences writer with a Master of Science (M.Sc.) degree from Lancaster University, UK. Before becoming a writer, Hannah's research focussed on the discovery of biomarkers for Alzheimer's and Parkinson's disease. She also worked to further elucidate the biological pathways involved in these diseases. Outside of her work, Hannah enjoys swimming, taking her dog for a walk and travelling the world.
Source: Read Full Article
