A team of investigators has discovered a genomic variant that may help clinicians predict which patients will experience cardiotoxicity from a widely used chemotherapy drug, according to a Northwestern Medicine study published in Circulation.
The findings underscore the significance of identifying reliable predictive genomic biomarkers and their potential to improve the course of treatment plans and overall patient outcomes, according to Paul Burridge, Ph.D., assistant professor of Pharmacology and senior author of the study.
“This is good news for patients because potentially we can predict which patients are more or least likely to experience cardiotoxicity and modify their treatment accordingly,” said Burridge, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Doxorubicin, an anthracycline chemotherapy drug, is mainly used as a last line of defense for patients diagnosed with cancer, most often pediatric cancers and breast cancers, who have no other chemotherapy treatment options.
While the drug has proven effective in slowing the growth of cancer cells, about 10 percent of patients will experience some form of cardiotoxicity from the drug, ranging from a reduction in ejection fraction—how well the heart’s left ventricle pumps blood—to heart failure, including requiring a heart transplant.
“Sadly, these patients with their previous history of cancer aren’t necessarily good candidates to be selected for a heart transplant,” Burridge said.
To pinpoint why doxorubicin causes cardiotoxicity in just a fraction of these patients, Burridge and his collaborators used previous work suggesting a variant in the gene SLC28A3 was statistically associated with doxorubicin cardiotoxicity to springboard their current study.
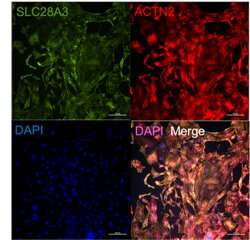
The SLC28A3 protein is also known as an uptake transporter that brings drugs into cancer cells and is only be expressed in cardiomyocytes, specialized cells that help the heart contract, according to Burridge.
With this knowledge in hand, the investigators engineered cardiomyocytes derived from human induced pluripotent stem cells (hiPSCs) from six pediatric patients. Half of these patients had the SLC28A3 variant and all patients had been treated with doxorubicin.
Using hiPSC-derived cardiomyocytes, the investigators found that cells from patients who had the SLC28A3 variant experienced less cardiotoxicity from the chemotherapy drug.
Knocking out SLC28A3 in cell lines also demonstrated a reduction in cardiotoxicity. However, the investigators also found that the SLC28A3 variant was a synonymous mutation and did not change the protein, further suggesting that another genomic variant was in fact responsible.
The investigators then developed a simple gene sequencing methodology using Nanopore DNA sequencing and identified a novel genomic variant in SLC28A3 called s11140490 that was located by long noncoding-RNA, having direct impact on SLC28A3 gene expression.
By simply screening patients for this variant, Burridge said, clinicians could be able to predict which patients will be protected from doxorubicin-induced cardiotoxicity. The study also establishes a model for screening clinically approved drugs for how well they transport doxorubicin into cardiomyocytes, according to Burridge.
Using this model, Burridge’s team found that the drug desipramine, a tricyclic antidepressant, inhibited the transport of doxorubicin into cardiomyocytes.
By studying mouse models treated with desipramine and then doxorubicin, the investigators found that cardiotoxicity in the mice was reduced by almost half.
“Desipramine is an already FDA-approved drug, so that means the route towards this therapy being used clinically is much shorter,” Burridge said.
Burridge said his team is now collaborating with the Lurie Cancer Center to begin testing desipramine’s efficacy in clinical trials, as well as to confirm that there are no issues with reducing the efficacy of chemotherapy.
Source: Read Full Article
