The existence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern that may better evade immunity established by vaccination or past infection by the wild-type form of the virus has been noted since early in the pandemic, with mutations to the receptor-binding domain and spike protein already having been seen to increase transmissibility in some lineages.
The duration of protection conferred by the currently available SARS-CoV-2 vaccines or previous infection, particularly in the face of newly developed variants of concern, has also not yet been clearly established given the insufficient time frame.
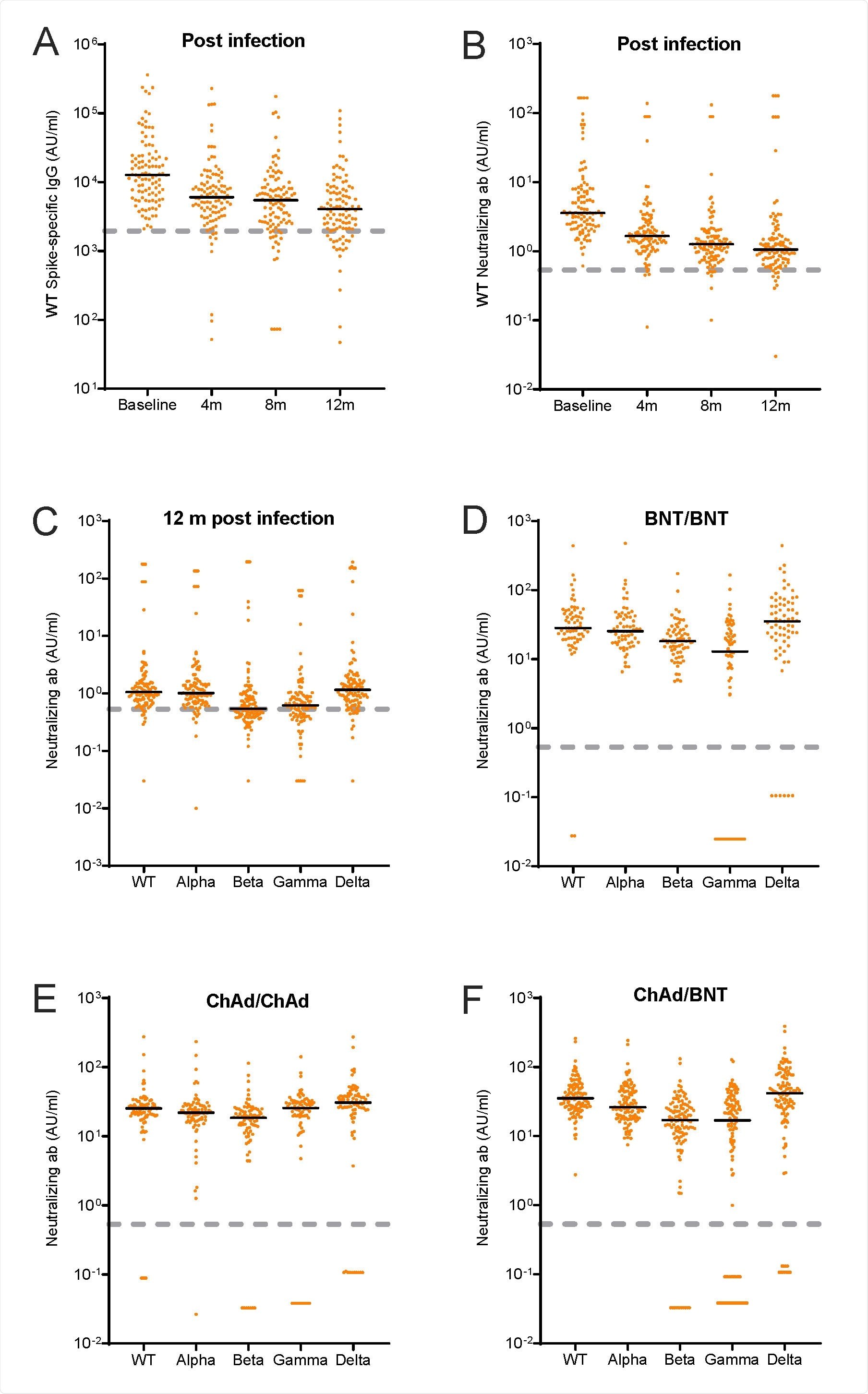
In a research paper recently uploaded to the preprint server medRxiv* by Havervall et al. (August 12th, 2021), neutralizing antibody titers towards wild-type SARS-CoV-2 and several variants of concern in individuals having either been vaccinated or infected with mild COVID-19 one year earlier are assessed, finding a reduced neutralization capacity against some strains.
-1.jpg)
How was the study performed?
The research group utilized data collected from over 2,000 healthcare workers in Sweden who were invited for repeated blood samplings every four months for a period of one year, having received either two doses of the Pfizer or AstraZeneca vaccine or mixed dosing of these vaccines.
An additional subset of participants had experienced mild COVID-19 the year before, confirmed by positive SARS-CoV-2 spike protein Immunoglobulin G (IgG) assay at the time, and had not yet received a vaccine.
In line with other reports on the lasting potency of neutralizing antibodies induced by both vaccines and past infection, the group note initially high levels of circulating SARS-CoV-2 spike protein IgG and neutralizing antibodies that decline over a few months, eventually plateauing to a roughly constant level.
Wild-type spike IgG was detected in 91%, 84% and 80% of participants at 4, 8, and 12 months, respectively, and wild-type neutralizing antibodies in 96%, 93%, and 91% of participants at the same time points.
As expected, high neutralizing antibody levels at the early stages post-vaccination or infection were associated with higher levels one year later.
Neutralizing antibody potency towards variants of concern
The group compared the neutralization capacity of the sera collected from individuals with one-year-prior COVID-19 experience against wild-type, B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta) strains, finding only slightly reduced potency against the alpha and delta strains. There was a more significant loss in neutralization capacity towards the beta and gamma strains, however.
Among the vaccinated participants, a similar loss of potency against the beta and gamma strains was observed by any vaccine type compared to the wild type.
Interestingly, a heterologous vaccination regime of the AstraZeneca adenovirus-based vaccine followed by the Pfizer mRNA-based vaccine also saw a reduced efficacy towards beta and gamma strains, though also with slightly reduced potency towards the alpha strain.
Mutations to the SARS-CoV-2 spike protein that alters the structure sufficiently to significantly reduce the affinity of neutralizing antibodies have been scarcely documented. However, some appear to have been identified, with the alpha variant bearing mutations in positions 417 and 484 and the gamma variant also bearing an alternative threonine in position 417 that may have a negative influence on antibody affinity.
Besides mutations that directly reduce the affinity of neutralizing antibodies towards the virus, mutations that increase transmissibility may also contribute towards the evasion of established immunity.
Mutations that increase the affinity of the spike protein towards the ACE2 receptor, as have been thoroughly reported in several variants of concern, including the delta strain, can significantly increase the virus's transmissibility by raising the probability of cell entry.
The group suggests that this strain's higher rate of proliferation caused any observed loss in antibody potency rather than any mutations that directly reduce the efficacy of neutralizing antibodies. Furthermore, in the case of notably higher transmission, fewer virions are needed for an infection to effectively establish, as each viral particle is more capable of initiating infection individually. Therefore it is more challenging to fend off infection, regardless of the potency of antibodies towards the virus, as each escape virion is more likely to establish infection.
The beta and gamma strains bear the E848 mutation to the spike protein that has been reported to enhance immune evasion amongst these strains. This study supports this, where the neutralization capacity of sera collected from both vaccinated COVID-experienced individuals was reduced compared to wild-type.
Overall the immunity induced by both vaccination and prior infection was still robust one year later, particularly against wild-type SARS-CoV-2 and the now more common alpha and delta strains, though potency towards the later-emerging beta and gamma strains is waning. In low vaccine availability locations, this is reassuring, as some protection can still be granted to individuals without committing to regular vaccine boosters in the short term.
However, the next generation of SARS-CoV-2 vaccines will need to account for mutations that allow evasion of the previously developed antibodies and preferably be better future-proofed against other novel mutations.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Neutralization of VOCs including Delta one year post-COVID-19 or vaccine, Sebastian Havervall, Ulrika Marking, Max Gordon, Henry Ng, Nina Greilert-Norin, Sarah Lindbo, Kim Blom, Peter Nilsson, Mia Phillipson, Jonas Klingstrom, Sara Mangsbo, Mikael Aberg, Sophia Hober, Charlotte Thalin, medRxiv, 2021.08.12.21261951; doi: https://doi.org/10.1101/2021.08.12.21261951, https://www.medrxiv.org/content/10.1101/2021.08.12.21261951v2
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Adenovirus, Antibodies, Antibody, Assay, Blood, Cell, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Healthcare, immunity, Immunoglobulin, Mutation, Next Generation, Pandemic, Proliferation, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Threonine, Vaccine, Virus

Written by
Michael Greenwood
Michael graduated from Manchester Metropolitan University with a B.Sc. in Chemistry in 2014, where he majored in organic, inorganic, physical and analytical chemistry. He is currently completing a Ph.D. on the design and production of gold nanoparticles able to act as multimodal anticancer agents, being both drug delivery platforms and radiation dose enhancers.
Source: Read Full Article
