Most early treatments against the coronavirus disease 2019 (COVID-19) have targeted the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, as it is required for viral entry into host cells. However, many SARS-CoV-2 variants exhibit significant mutations in the spike protein, thus reducing the effectiveness of many vaccines and monoclonal antibody treatments that target this antigen.
In a recent study published on the preprint server bioRxiv*, researchers discuss alternate therapeutic targets for treating COVID-19 and the effects of Gal-9 on SARS-CoV-2 infection and the immune response.
Study: Human Galectin-9 Potently Enhances SARS-CoV-2 Replication and Inflammation In Airway Epithelial Cells. Image Credit: Corona Borealis Studio / Shutterstock.com
Study findings
In the current study, Calu-3 human airway epithelial cells (AECs) were dosed with recombinant Gal-9 to determine the 50% cytotoxic concentration (CC50) using the MTT assay. Calu-3 cells were then dosed with 50 nanomolar (nM), 100nM, or 250nM Gal-9 for six hours before viral infection with SARS-CoV-2. The cells then remained in the media for 24 hours before SARS-CoV-2 infection was measured by quantitation of nucleocapsid (N) gene expression.
As the Gal-9 concentration increased, SARS-CoV-2 infection increased significantly in a dose-dependent manner, with the highest concentrations showing up to 27-fold increases. The release of infectious virus into the supernatant increased in a similar manner, as measured by the median tissue culture infectious dose (TCID50). Immunofluorescence assays with specific staining of the N protein confirmed the enhancement of virus production by Gal-9.
The researchers then investigated the specific stage of the replication site impacted by Gal-9 by examining Calu-3 cells treated with 350 nM Gal-9 before and after SARS-CoV-2 infection. To this end, pre-treated cells showed significantly higher virus production, thus suggesting that Gal-9 impacts the early stage of the viral life cycle.
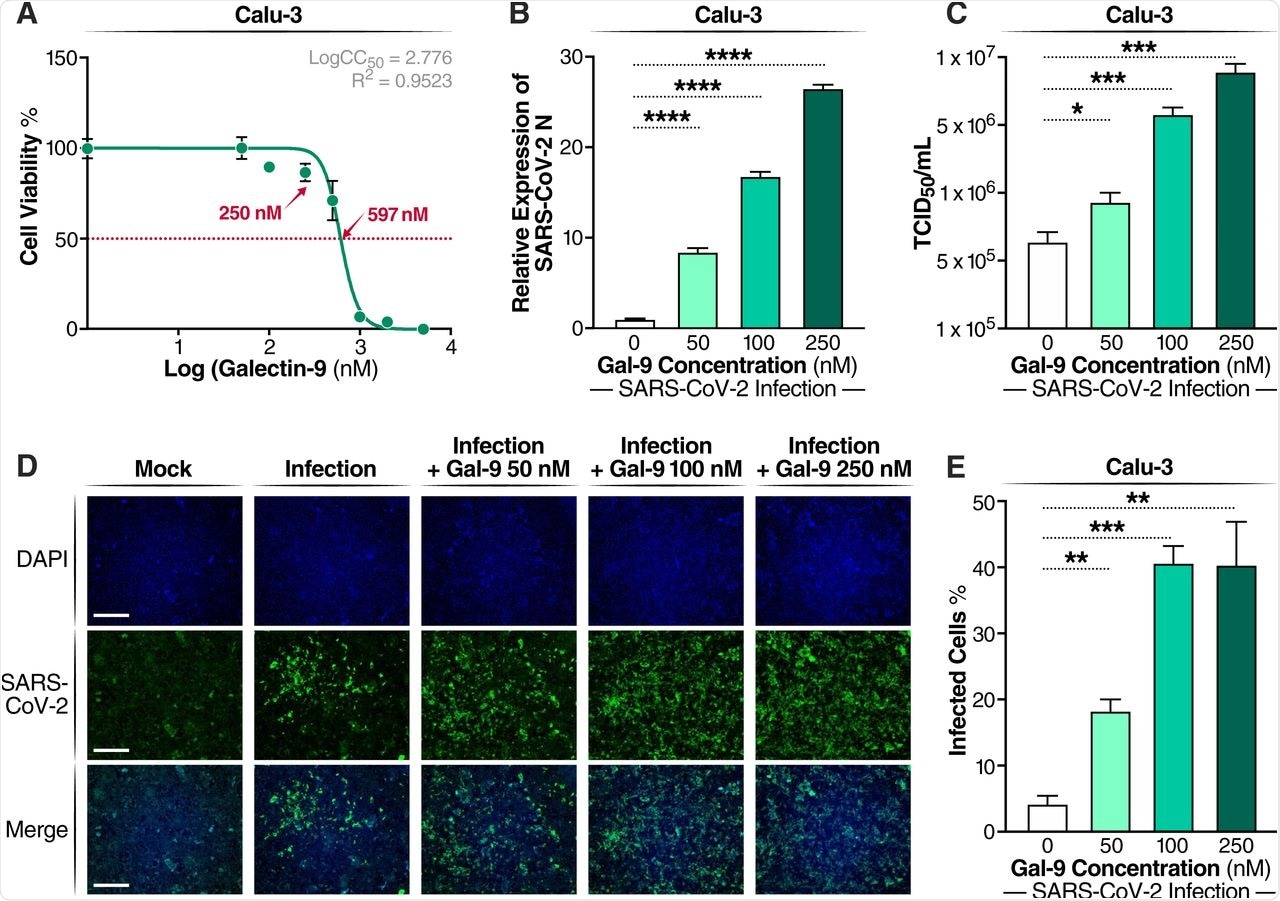
Gal-9 increases virus production in SARS-CoV-2-infected Calu-3 cells. (A) Cellular toxicity was examined in Calu-3 cells using an MTT assay and was expressed as relative cell viability as compared to Gal-9-untreated control (set at 100%). The LogCC50 value for Gal-9 is displayed. The red arrows represent 250 nM and the CC50 value (597 nM) of Gal-9, respectively. (B) The effect of Gal-9 on viral N gene expression in Calu-3 cells was measured by RT-qPCR. Cells were pretreated with Gal-9 at the indicated concentrations for six hours, followed by infection with SARS-CoV-2 (MOI=0.01) for 24 h in the presence of Gal-9. 24 hpi, cells were collected for RNA isolation and RT-qPCR targeting the N gene. (C) Infectious virus release in the supernatant of SARS-CoV-2-infected Calu-3 cells treated with varying doses of Gal-9 as described in (B) were measured using TCID50. (D) Immunofluorescence staining of Calu-3 cells with DAPI (blue) or anti-N Ab (green) pretreated with Gal-9 at the indicated concentrations, followed by infection with SARS-CoV-2 as described in (B). Scale bar, 500 μM. (E) Quantification of SARS-CoV-2 infected cells in Calu-3 cells (shown in panel D). Data are representative of the results of three independent experiments (mean ± SEM). Statistical significance was analyzed by t test. p≤0.05 [*], p≤0.01 [**], p≤0.001 [***], p≤0.0001 [****].
Cells were then incubated at low temperature for two hours, followed by the detection of any attached particles after triple washing of the cells. Gal-9 treatment showed substantial increases in cell attachment in a dose-dependent manner.
To determine if this observation was dependent on the angiotensin-converting enzyme 2 (ACE2) receptor, cells were treated with a competitive anti-ACE2 antibody shown to block SARS-CoV-2 pseudovirus infection. This agent successfully blocked Gal-9 enhanced infection as well.
To determine the mechanisms responsible for the enhancement of infection, cell surface ACE2 and transmembrane serine protease 2 (TMPRSS2) expression were measured by flow cytometry. To this end, Gal-9 did not appear to affect the expression of ACE2 nor TMPRSS2.
Sandwich enzyme-linked immunosorbent assay (ELISA) was then used to examine the binding between ACE2 and the spike protein. This experiment showed significantly enhanced binding when cells were incubated with Gal-9.
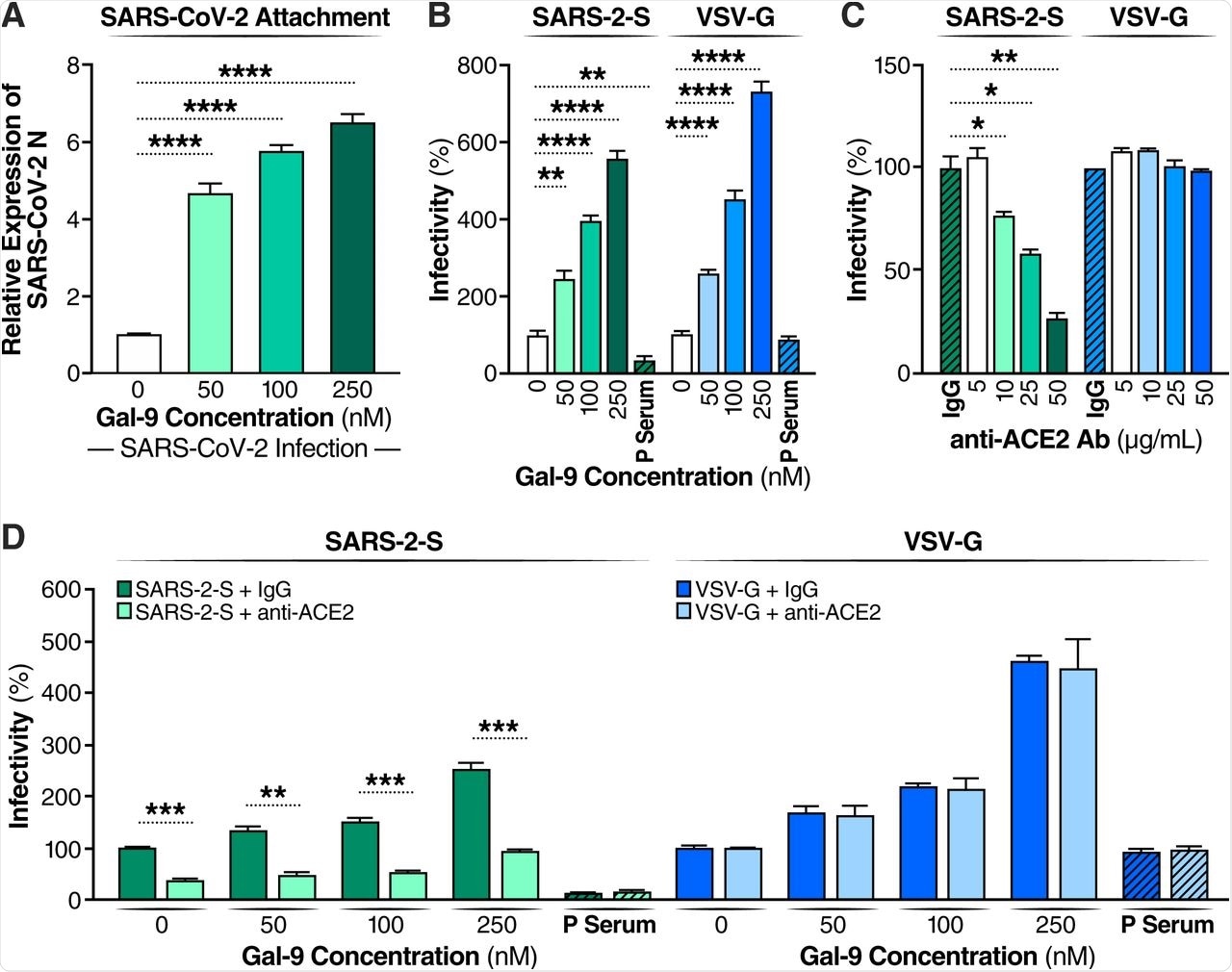
Gal-9 facilitates the cellular attachment and entry of SARS-CoV-2. (A) Attached SARS-CoV-2 virions on the cell surface were detected by RT-qPCR. Calu-3 cells were pretreated with Gal-9 for one hour, then cells were incubated with SARS-CoV-2 (MOI=0.01) in solutions with or without Gal-9 at the indicated concentrations at 4°C for two hours. Cells were washed three times with PBS and harvested for RNA isolation and RT-qPCR measurement of SARS-CoV-2 N gene expression. (B) Relative infectivity of SARS-2-S pseudotyped virus and VSV-G pseudotyped virus in Calu-3 treated with Gal-9 at the indicated concentrations. Calu-3 cells were exposed to Gal-9 for six hours and then infected with SARS-2-S pseudotyped virus or VSV-G pseudotyped virus in solutions containing Gal-9 at the indicated concentrations. Pseudotyped viral entry was analyzed by luciferase activity 24 hpi. Positive serum predetermined to possess anti-SARS-CoV-2 neutralizing activity was used as a negative control. Luciferase signals obtained in the absence of Gal-9 were used for normalization. (C) Relative infectivity of SARS-2-S pseudotyped virus and VSV-G pseudotyped virus in Calu-3 cells treated with anti-ACE2 Ab at the indicated concentrations. Calu-3 cells pre-incubated with anti-ACE2 Ab (R&D Systems, AF933) at the indicated concentrations, or control antibody (anti-goat IgG (R&D Systems, AB-108-C), 50 μg/ml) were co-administered with SARS-2-S pseudotyped and VSV-G pseudotyped virus. At 24 hpi, pseudotyped viral entry was analyzed by luciferase activity. Luciferase signals obtained using the control antibody (anti-goat IgG, 50 μg/ml) were used for normalization. (D) The effect of anti-ACE2 Ab on Gal-9-enhanced cell entry of SARS-2-S was evaluated by measuring luciferase activity. Calu-3 cells were pretreated with Gal-9 for six hours and then pre-incubated with anti-ACE2 Ab (25 μg/ml) or control antibody for one hour, and cells were inoculated with SARS-2-S pseudotyped or VSV-G pseudotyped virus in a solution containing Gal-9 at the indicated concentrations. At 24 hpi, pseudotyped viral entry was analyzed by luciferase activity. Luciferase signals obtained in the absence of both Gal-9 and anti-ACE2 Ab were used for normalization. Data are representative of the results of three independent experiments (mean ± SEM). Statistical significance was analyzed by t test. p>0.05 [ns], p≤0.05 [*], p≤ 0.01 [**], p≤0.001 [***], p≤0.0001 [****].
When examining the growth curves of the enhanced replication, expression of the N gene increased significantly within one to three hours post-infection (hpi) in Calu-3 cells as compared to untreated controls. Maximum viral yields in treated cells were detected at 36 hpi as compared to 48 hpi in untreated cells.
Microscopy images showed that SARS-CoV-2-mediated cytopathic effects were also much more pronounced in treated cultures. As SARS-CoV-2 is commonly associated with immune dysfunction, the immune response in both treated and untreated cells revealed that Gal-9 induced the expression of interleukin 6 (IL-6), IL-8, and tumor necrosis factor α (TNF-α) to modest levels before SARS-CoV-2 infection.
However, if the cells were infected with SARS-CoV-2, these same immune responses were induced to much higher levels at earlier time frames. Without Gal-9 treatment, IL-6, IL-8, and TNF-α were induced at 24, 26, and 24 hpi, respectively; however, treated cells showed similar levels at 9, 12 and 9 hours.
Ribonucleic acid sequencing (RNA-seq) analysis on Calu-3 cells infected for 24 hours with or without Gal-9 treatment was used to better understand the transcriptional impact of Gal-9. Differentially expressed gene analysis revealed that only one protein-coding gene of RNU12 showed significantly altered expression following SARS-CoV-2 alone. Gal-9 treatment without infection caused significant modulation of 87 genes. Cells infected in the presence of Gal-9 experienced a significant change in their transcriptome, with 1,094 differentially expressed genes.
Ingenuity Pathway Analysis allowed the enriched pathways corresponding to these genes to be analyzed. This revealed that several pro-inflammatory proteins and pathways were activated in the presence of infected cells treated with Gal-9 including IL-6, IL-8, and JAK-STAT signaling pathways.
Finally, AECs cultured at the air/liquid interface (ALI) were pre-treated with Gal-9 and then infected with SARS-CoV-2 for 36 hours. Productive infection could be seen in all cultures from five different donors. Furthermore, real-time polymerase chain reaction (RT-PCR) revealed that IL-6 expression was induced after infection in treated cells.
Conclusions
The researchers of the current study have demonstrated that Gal-9 enhances the ability of SARS-CoV-2 to replicate within cells and that this is effect likely, at least in part, due to the enhanced spike protein-ACE2 binding. The current study has found that Gal-9 treated cells show higher levels of immune response much earlier than non-treated cells and exhibited significantly altered expression of immunologically active genes.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information
- Du, L., Bouzidi, M. S., Gala, A., et al. (2022). Human Galectin-9 Potently Enhances SARS-CoV-2 Replication and Inflammation In Airway Epithelial Cells. bioRxiv. doi:10.1101/2022.03.18.484956
Posted in: Drug Discovery & Pharmaceuticals | Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibody, Antigen, Assay, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytometry, Drug Discovery, Enzyme, Flow Cytometry, Gene, Gene Expression, Genes, Immune Response, Inflammation, Interleukin, Luciferase, Microscopy, Monoclonal Antibody, Necrosis, Polymerase, Polymerase Chain Reaction, Protein, Pseudovirus, Receptor, Respiratory, Ribonucleic Acid, RNA, SARS, SARS-CoV-2, Serine, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Tissue Culture, Tumor, Tumor Necrosis Factor, Virus

Written by
Sam Hancock
Sam completed his MSci in Genetics at the University of Nottingham in 2019, fuelled initially by an interest in genetic ageing. As part of his degree, he also investigated the role of rnh genes in originless replication in archaea.
Source: Read Full Article
