Researchers in the UK have developed a high-throughput standardizable assay that accurately detects T cell responses to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent that causes coronavirus disease 2019 (COVID-19).
Andrew Godkin from Cardiff University and colleagues say that such tests are imperative for understanding the role that T cell responses play in long-term immunity against SARS-CoV-2, particularly among immunocompromised individuals.
“Whilst the exact nature of effective immunity remains incompletely defined, SARS-CoV-2-specific T cell responses are a critical feature of the immune response that will likely form a key correlate of protection against COVID-19,” writes the team.
The ability to rapidly identify the presence of SARS-CoV-2 -specific T cell responses would help to determine the adaptive immune status of previously infected or vaccinated individuals and detect unsuspected previous infection.
The researchers say that including such data in population immunity studies or personalized immunity certificates could have far-reaching implications for lockdown policies.
This information could help to improve the assessment of vaccine effectiveness in communities and highlight the potential need for booster doses in cases where immunity has waned.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.
.jpg)
“Effective COVID-19 immunity at a population level must be understood”
Researchers are increasingly trying to understand how the adaptive immune responses generated following SARS-CoV-2 infection or vaccination might protect against future infection.
“In order to control future outbreaks and identify at-risk individuals, the exact constituents of effective COVID-19 immunity at a population level must be understood,” says Godkin and colleagues.
Multiple studies have recently shown that virus-specific T cell responses develop in almost every case of SARS-CoV-2 infection and persist for at least six months.
Traditionally, viral antigen-specific T cell responses have been assessed using flow cytometry or ELISpot-based readouts. However, neither approach is amenable to high-throughput processing or is standardizable across laboratories.
Furthermore, currently available assays that detect SARS-CoV-2-specific cellular immune responses only measure the cytokine interferon-gamma (IFNg) that is released by T cells, despite other Type 1 T helper (TH1) cell-type cytokines potentially being better indicators of anti-viral responses.
To overcome these limitations, existing whole blood-based immunoassays can be adapted to measure virus-specific T cell responses in a high-throughput, standardizable manner.
Monitoring immune responses in the elderly and immunosuppressed is particularly important
Monitoring immune response to SARS-CoV-2 amongst elderly and immunosuppressed individuals is particularly important, given the significantly higher mortality rates observed in these groups.
The incidence of cancer is also increased among elderly individuals, where declining adaptive immune responses and age-associated inflammation contribute to disease progression.
“Indeed, early indications suggest that cancer patients, in particular those on active treatments such as chemotherapy, were significantly less likely to mount antibody and T cell responses to the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine,” says Godkin and the team.
What did the researchers do?
The researchers adapted and optimized a widely utilized, high-throughput whole blood-based assay to determine the TH1-type (IFNg/IL-2) cellular immune responses associated with previous SARS-CoV-2 infection and/or vaccination among 156 healthy donors and 67 solid-organ cancer patients.
Participants were recruited between February and April 2021 and were stratified based on their self-reported and/or laboratory evidence of a previous SARS-CoV-2 infection. Participants reporting no prior positive test were defined as “unknown/naïve.”
To measure the immune responses generated by COVID-19 vaccination, blood samples were taken immediately before the first dose and 3 to 6 weeks later.
All immunized individuals received either Pfizer-BioNTech’s BNT162b2 vaccine or AstraZeneca’s ChAdOx1 nCoV-19 vaccine.
What did the study find?
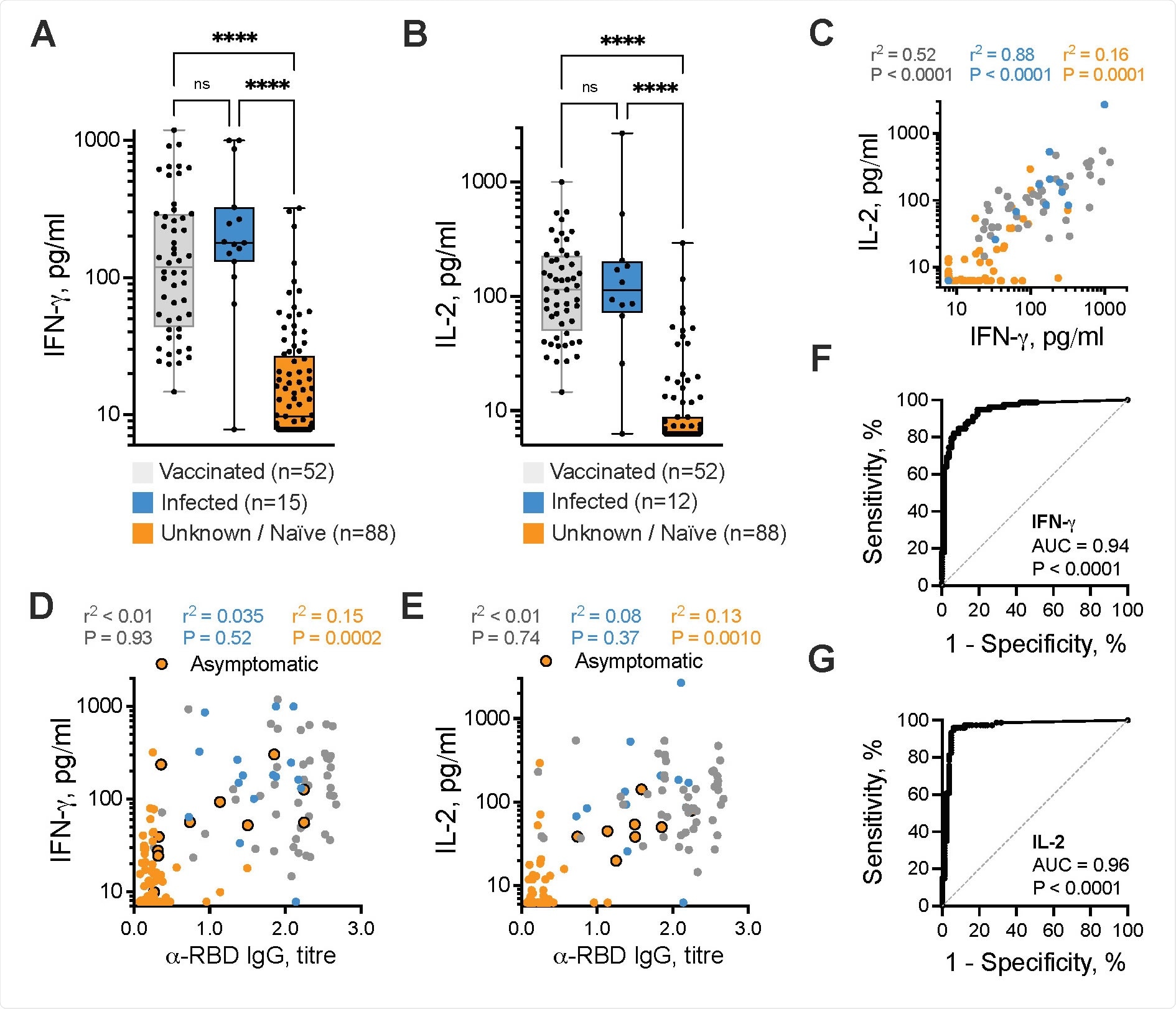
Among the healthy donors, significant differences in IFNg+ and IL-2+ SARS-CoV-2-specific T cell responses were observed between vaccinated or previously infected individuals and the unknown/naïve individuals.
The measurement of IL-2 generated by T cells in response to SARS-CoV-2-derived antigens was a highly predictive diagnostic assay, with a sensitivity of 96.0% and a specificity of 93.9%.
Measurement of IFNg+ SARS-CoV-2 specific T cell responses was also effective at identifying infected participants who were asymptomatic.
A single dose of vaccine-induced IFNg and/or IL-2 SARS-CoV-2-specific T cell responses in 28 (96.6%) of 29 healthy donors, but only in 27 (48.2%) of 56 cancer patients.
“These data provide further support to recent calls for cancer patients to be prioritized for booster vaccines and longer-term immunological monitoring,” writes the team.
What did the authors conclude?
The researchers say that this cost-effective, high-throughput standardizable test enabling accurate and comparable assessment of SARS-CoV-2-specific T cell responses would be amenable to widespread population immunity testing.
“We demonstrate high sensitivity and specificity of this assay to identify or exclude prior SARS-CoV-2 infection and/or successful COVID-19 vaccination,” says Godkin and colleagues.
“Going forward, it is imperative to utilize such tests to understand the precise contribution of T cell responses with regards to long-term immunity to SARS-CoV-2 infection, in particular amongst immunocompromised individuals,” they conclude.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Godkin A, et al. Whole blood-based measurement of SARS-CoV-2-specific T cell responses reveals asymptomatic infection and vaccine efficacy in healthy subjects and patients with solid organ cancers. medRxiv, 2021. doi: https://doi.org/10.1101/2021.06.02.21258218, https://www.medrxiv.org/content/10.1101/2021.06.02.21258218v1
Posted in: Device / Technology News | Medical Research News | Disease/Infection News
Tags: Antibody, Antigen, Assay, Blood, Cancer, Cell, Chemotherapy, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Cytokines, Cytometry, Diagnostic, Efficacy, Flow Cytometry, Immune Response, Immunoassays, Inflammation, Laboratory, Mortality, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article
