The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a mammoth public health challenge. COVID vaccines have helped curb the pandemic; however, vaccine effectiveness depends on the duration of antibody responses they elicit.
A new study has assessed the antibody responses to the mRNA-1273 Moderna vaccine over time. The investigators have evaluated the anti-SARS-CoV-2 antibody responses from blood samples of individuals prior to vaccination up to 6.5 months after vaccination. A preprint version of the study, which is yet to undergo peer review, is available on the medRxiv* server.
 Study: A longitudinal seroconversion panel shows anti-SARS-CoV-2 antibody levels up to 6.5 months after vaccination with mRNA-1273 (Moderna). Image Credit: Jonathan Weiss / Shutterstock
Study: A longitudinal seroconversion panel shows anti-SARS-CoV-2 antibody levels up to 6.5 months after vaccination with mRNA-1273 (Moderna). Image Credit: Jonathan Weiss / Shutterstock
Vaccines
Since the declaration of the COVID-19 pandemic, several efforts have been taken to combat the virus. To contain COVID-19, a wide range of approaches have been used, including passive immunity through convalescent plasma, monoclonal antibodies, pharmaceuticals, and vaccines. Different vaccine technologies have been developed, including mRNA, DNA, protein, and non-replicating virus technologies.
mRNA-1273 Moderna vaccine
mRNA-1273 vaccine by Moderna is an advanced vaccine based on messenger RNA (mRNA) and nanotechnology. Today, the U.S. Food and Drug Administration approved the Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax to prevent COVID-19 in individuals 18 years of age and older. Moderna’s COVID-19 Vaccine had been available under emergency use authorization (EUA) for individuals 18 years of age and older since Dec. 18, 2020. This vaccine is given in two doses. Each dose consists of 100 µg pre-fusion stabilized spike protein mRNA. The second dose is administered four weeks after the first dose.
A third dose called the booster is also authorized by EUA for individuals aged 18 and above and immunocompromised individuals that are at a high risk of infection.
Seroconversion panels
This study enrolled 15 volunteer healthcare workers – nine male and six female. All participants were white/Caucasian adults 30-60 years of age. Participants had received two doses of the mRNA-1273 vaccine 28 days apart. The investigators collected blood samples from the participants at different time intervals. Sample 1 was a pre-vaccination sample collected 0 to 3 days before the first dose.
Sample 2 was collected 2 to 7 days before the second vaccination. Sample 3 was collected 13 to 15 days after the second vaccination. Sample 4 was collected 41 to 45 days or 1.5 months after the second vaccination. Sample 5 was collected 103 to 107 days or 3.5 months after the second vaccination. Sample 6 was collected 194 to 199 days or 6.5 months after the second vaccination. The samples were tested for anti-SARS-CoV-2 antibody activity using a chemiluminescent immunoassay. This will provide anti-SARS-CoV-2 antibody responses for constructing longitudinal seroconversion panels. Seroconversion panels help in assessing antibody responses over time.
Dynamic changes in antibody responses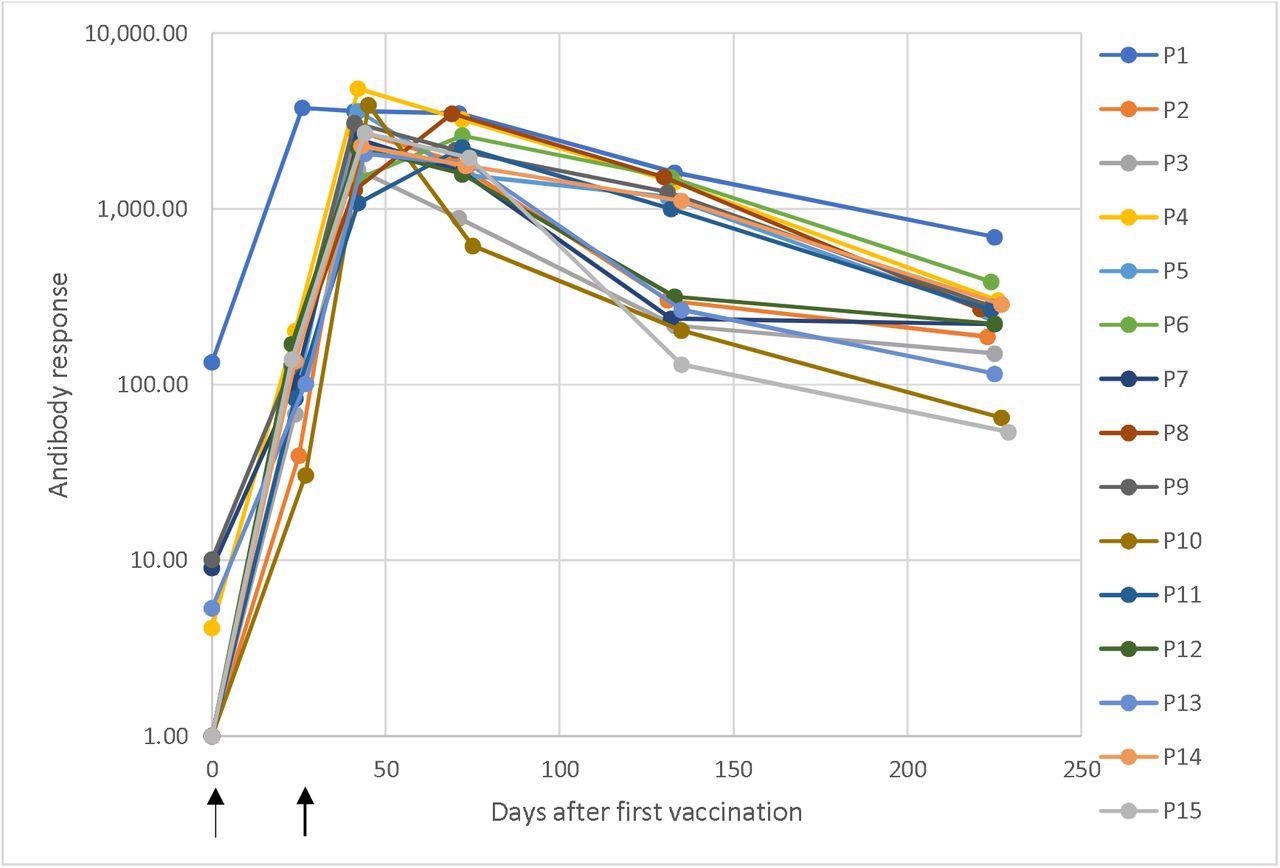
Dynamic changes in antibody response against SARS-CoV-2 in a longitudinal seroconversion panel from 15 participants (P1–P15) before and after vaccination with two doses of the mRNA-1273 SARS-CoV-2 vaccine (Moderna), indicated by arrows. The sample was collected prior to vaccination and up to 6.5 months from the second vaccine dose. These results were obtained using a chemiluminescent immunoassay and are expressed as arbitrary units/mL (AU/mL). Responses ≥ 15.0 units were considered positive. Time 0 corresponds with the sample obtained before the first vaccination dose and the time of the subsequent samples are the elapsed days after the pre-vaccinated sample.
The longitudinal seroconversion panel from 15 participants demonstrated dynamic changes in antibody responses against SARS-CoV-2. One of the participants tested positive for anti-SARS-CoV2 antibodies before vaccination. This indicated a prior infection. The rest of the participants tested negative for anti-SARS-CoV-2 antibodies before vaccination.
All 15 participants had a positive antibody response after receiving the first dose of the vaccine. The antibody responses peaked and were the highest after the second dose. After this peak, the antibody responses slowly and steadily declined with time.
Conclusion
All participants showed positive antibody responses after the first dose of the vaccine. This robust response is enhanced after the second dose of the vaccine. The participants also showed positive antibody responses even after 6.5 months after the second dose. However, the levels had declined over time.
This study concurs with previous studies that have demonstrated declining antibody responses in the months after vaccination with mRNA-based vaccines.
Another study has also shown that there is a slight increase in SARS-CoV-2 infections in mRNA-1273 vaccinated individuals 4-5 months after vaccination. However, the rate of infection in unvaccinated participants is much higher than the infection rate in vaccinated participants. Furthermore, one in ten unvaccinated individuals had to be hospitalized. The hospitalization frequency is far less for vaccinated individuals. The number of deaths due to COVID-19 is also far less in vaccinated individuals than the unvaccinated individuals.
This study supports the administration of a booster dose to increase antibody levels 4-6 months after the initial two doses. It also advocates continued monitoring to assess the durability of COVID-19 vaccine responses.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Belda F, Mora O, Christie R, Crowley M. A longitudinal seroconversion panel shows anti-SARS-CoV-2 antibody levels up to 6.5 months after vaccination with mRNA-1273 (Moderna). medRxiv. January 2022:2022.01.25.22269762. doi:10.1101/2022.01.25.22269762, https://www.medrxiv.org/content/10.1101/2022.01.25.22269762v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Blood, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, covid-19, DNA, Food, Frequency, Healthcare, immunity, Immunoassay, Nanotechnology, Pandemic, Pharmaceuticals, Protein, Public Health, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Dr. Shital Sarah Ahaley
Dr. Shital Sarah Ahaley is a medical writer. She completed her Bachelor's and Master's degree in Microbiology at the University of Pune. She then completed her Ph.D. at the Indian Institute of Science, Bengaluru where she studied muscle development and muscle diseases. After her Ph.D., she worked at the Indian Institute of Science, Education, and Research, Pune as a post-doctoral fellow. She then acquired and executed an independent grant from the DBT-Wellcome Trust India Alliance as an Early Career Fellow. Her work focused on RNA binding proteins and Hedgehog signaling.
Source: Read Full Article
