Karl Fischer titration is the most widely used technique to determine the water content in a sample. It is used in the analysiz of foods, pharmaceuticals and many chemicals, among others.
 Image Credit: Rattiya Thongdumhyu / Shutterstock
Image Credit: Rattiya Thongdumhyu / Shutterstock
Karl Fischer titration is based upon the original Bunsen reaction:
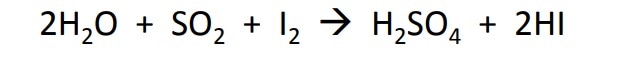
which was later modified to the final equation:
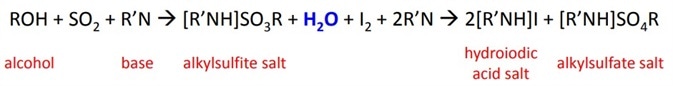
The reaction titrates the volume of KF reagent required to react the iodine until all the water in the sample is completely used up, at which point the excess iodine is detected by the indicator. The water is used up during the oxidation of the alkyl sulfite intermediate by iodine. The reaction is pH sensitive.
KF titration is performed either volumetrically or coulometrically. In the volumetric method, the iodine is added by mechanical means to the alcohol solvent in the titration cell containing the sample. The end-point of the titration is reached when the iodine stops reaction. Thewater content of the sample is calculated from the volume of the KF reagent used.
The volumetric titration is capable of titrating from 100 to 1×106 ppm (water concentration from 0.01 – 100%). However, it is preferred in samples with water content above 1%, or between 0.1 and 500 mg of water (typically 0.5 to 50 mg water).
Single- or two-component reagent?
The volumetric KF titration may be performed using either a single-component or two-component reagent. In the first case, the titrator buret contains a nonreactive solvent in which all the reagents required for the titration are dissolved.
In most cases this is methanol, which is pretitrated using the KF reagent to eliminate all contaminating water. Following this the sample is added and the automatic determination of its water content is carried out.
When a two-component reagent is selected, the titrant consists of iodine (already measured) dissolved in a solvent, which is added to the titrator buret until it is full. The KF solvent is now mixed with the sulfur dioxide and base and the solution is added to the titration cell.
This is also pre-titrated till all water is removed, before sample addition and water determination. The first method can be adapted to many workflows, using a variety of media. The second has greater speed of titration and polarity. Volumetric KF titration is typically capable of determining 0.5 to 50 mg water.
The volumetric KF reagents must be calibrated regularly as their water equivalents change over time. This is done using certified water standards for convenience. Water equivalent is the amount of water taken up by 1 ml of reagent.
Components of automated KF titration
KF titration has been fully automated today. The determination of the water content is performed at the press of a button on the titrator and the result is read out or printed out.
A motorized buret with a titration stand to ensure accurate doses of the KF reagent are added even in the microliter range. To enable this 10 000 steps have been provided in the mechanical buret.
An exchange unit for reagent dosing, comprising a cylinder, buret, tap, buret tip and the container of KF reagent, the last being equipped with a drying tube to prevent the entry of atmospheric moisture.
These sample exchangers have sample beakers into which weighed samples are added, closed with aluminum foil against atmospheric moisture, placed ready for titration and then pierced to add a preset volume of solvent.
Once the required time for dissolution of extraction has passed, the KF titration begins. These help process numbers of samples without overburdening the staff, and can work without supervision, thus increasing the throughput.
An indicator system, using a bi-voltammetric indication system that has a double platinum electrode through which a constant current such as 50 µA passes. The reaction proceeds as long as there is water in the sample within the titration cell.
The cell needs to be kept at high voltage in order to sustain the polarization current of the electrode at the required preset level. Once the titration is complete, free iodine will be found in the solution, which leads to a voltage drop and thus the polarization current level.
Controls to ensure the fastest possible addition of the titration to the titration solution but to stop the addition as soon as the end point is achieved. This is automatically achieved in modern KF titrators by the self-adjustment of the control to the titration curve. This precise level of control allows the titration to be completed within minutes but with great accuracy.
Inbuilt communications including a keypad and monitor are used for both input (control parameters, sample data) and output, which includes the display of a live titration curve in the most advanced models, allowing the user to visualize the course of the titration.
Storage capacity is essential to keep the method quick and reliable, by saving each preset operating parameter set to memory. The ideal conditions of titration can be saved when multiple different samples require analysis. Once this is done the determination can be performed rapidly by simply selecting the right sample, adding it and beginning the process.
Interfaces are important to incorporate the KF titrator into the laboratory data management setup.
Advantages of volumetric Karl Fischer
The volumetric KF titration has several benefits for the user, such as:
- Selectivity in determining only water content
- Accuracy of final determination
- Capability to determine sample water over a wide range of water content
- Speed of determination
Applications of volumetric Karl Fischer
Volumetric KF titration has a range of applications, such as determining water content in:
- Chemicals
- Pharmaceuticals
- Petroleum and plastics
- Power stations
- Foodstuffs and animal feed
- Adhesives and paints
In all of these the water content is important as it determines the shelf life, the vulnerability to corrosion, many chemical properties, and the quality, each of these being vital for quality control in one product or the other.
Sources
- chem.uiowa.edu/…/LUCIO%20GM%20KF-Titration%20March-2013.pdf
- dmsc2.dmsc.moph.go.th/…/Karl%20Fischer%20Titration.pdf
- www.labicom.cz/…/HYDRANAL-Seminar-2016_Praha_Brno.web_.pdf
- www.gpsil.co.uk/…/#
Further Reading
- All Karl Fischer Titration Content
- What is Karl Fischer Titration and How Does It Work?
- Advantages and Limitations of Karl Fischer Titration
- What is the Difference Between Coulometric and Volumetric Titration?
- What is the Difference Between Titration and Karl Fischer Titration?
Last Updated: Feb 26, 2019

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article
