A team of international scientists has developed an mRNA-based coronavirus disease 2019 (COVID-19) vaccine encoding the receptor-binding domain (RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) delta variant. The vaccine candidate has shown high efficacy in inducing neutralizing antibodies and protecting against delta infection in mice. The study is currently available on the bioRxiv* preprint server.
 Study: A mRNA vaccine encoding for a RBD 60-mer nanoparticle elicits neutralizing antibodies and protective immunity against the SARS-CoV-2 delta variant in transgenic K18-hACE2 mice. Image Credit: NIAID
Study: A mRNA vaccine encoding for a RBD 60-mer nanoparticle elicits neutralizing antibodies and protective immunity against the SARS-CoV-2 delta variant in transgenic K18-hACE2 mice. Image Credit: NIAID
Background
The ongoing COVID-19 pandemic caused by SARS-CoV-2 has resulted in more than 477 million infections and over 6.1 million deaths worldwide. The vaccines developed at the later phase of the pandemic have shown high efficacy in preventing SARS-CoV-2 infection, symptomatic COVID-19, hospitalization, and mortality. Because of the global mass vaccination campaigns, an initial drop in COVID-19 cases has been observed in many countries. However, with the emergence of novel viral variants, including delta and omicron, a significant reduction in vaccine efficacy has been observed globally. A sharp rise in vaccine breakthrough cases has prompted public health authorities of many countries to introduce a third booster dose to the at-risk population to curb viral transmission. The development of new vaccines against emerging viral variants has also been advised.
In the current study, the scientists have developed and validated an mRNA-based vaccine that encodes an engineered nanoparticle displaying delta RBD as an immunogen. Specifically, a protein nanoparticle scaffold comprising 60 identical subunits of the self-assembling bacterial protein lumazine synthase has been used to display multimeric RBD of the SARS-CoV-2 delta variant.
To ensure effective delivery of the vaccine candidate, the scientists have complexed the mRNA construct with protamine, a small arginine-rich DNA-binding nuclear protein. Protamine has been widely used to condense and protect RNAs from degradation and prepare self-adjuvanted vaccines.
Humoral immune response induced by vaccine
The vaccine-induced antibody response was measured by intramuscularly injecting the mice with two doses at week 0 (prime) and week 3 (booster). The serum samples were collected from immunized mice before the first and second doses and three weeks after the second dose to measure neutralizing antibody titers.
The findings revealed that all immunized mice have high titers of neutralizing antibodies targeting the delta RBD at week 3 before administering the second vaccine dose. The samples collected 3 weeks after the second dose showed significantly higher antibody titers.
The virus neutralization assay was conducted using the authentic SARS-CoV-2 delta variant to assess the infection-protecting efficacy of vaccine-induced antibodies. Serum samples collected from human patients immunized with the two-dose regimen of mRNA-based Pfizer-developed COVID-19 vaccine were used as a positive control.
The findings revealed that antibodies developed 3 weeks after the second dose could significantly neutralize delta infection. The antibodies produced at week 3 (before administration of the second dose) also showed high neutralizing potency in some mice. The average antibody titer 3 weeks after the second dose was 13-fold higher than that induced by the Pfizer vaccine.
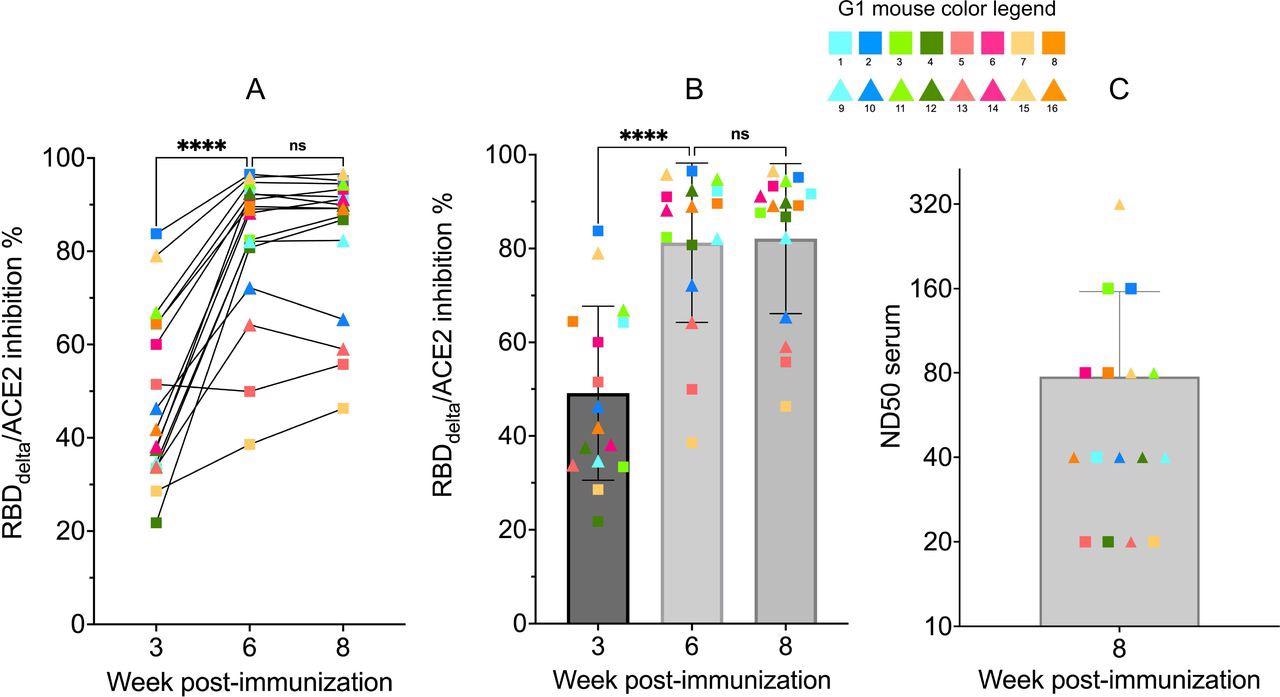
RBDdelta-ACE2 interaction inhibition by neutralizing antibodies and SARS- CoV-2 delta variant neutralization in K18-hACE2 transgenic mice Sera samples of immunized K18-hACE2 transgenic female mice (n= 16) analyzed with the SARS-CoV-2 neutralization antibody assay and the SARS-CoV-2 delta variant neutralization test (Group 1 with individual color code, squares indicate mice euthanized at 3 dpi and triangles indicate mice followed until 14 dpi). (A) RBDdelta-ACE2 interaction inhibition rate at week 3, 6 and 8 with paired individual values. (B) RBDdelta-ACE2 interaction inhibition rate at week 3, 6 and 8. Week 3 and 6 were significantly different in one-way ANOVA multiple comparison (p- value< 0.0001). Week 6 and 8 were not significantly different (p-value= 0.66). (C) Median neutralization dose (ND50) at week 8.
Protective efficacy of the vaccine
The vaccine's protective efficacy against delta infection was assessed in transgenic mice expressing human angiotensin-converting enzyme 2 (ACE2). The mice were immunized with the vaccine using the same vaccination regimen. Five weeks after full immunization, all mice were challenged intranasally with SARS-CoV-2 delta variant and followed up for 14 days post-infection.
Compared to unvaccinated control mice that showed severe symptoms and rapid bodyweight loss, all immunized mice exhibited body weight gain and occasional mild symptoms. Compared to unvaccinated mice, all immunized mice also showed significantly lower lung viral load and infectious viral titer at day 3 post-infection.
Regarding cellular immune response, immunized mice showed significantly lower expression of pro-inflammatory cytokines and chemokines compared to unimmunized mice at day 3 post-infection. In addition, significantly elevated expressions of CCL5 and IL12 were observed in immunized mice. Both IL12 and CCL5 play essential roles in inducing T cell proliferation, expansion, and activation. Taken together, these observations indicate that the vaccine is capable of generating robust and durable cellular immune responses against the delta variant.
Study significance
The study describes the development and validation of an mRNA-based nanoparticle-formulated COVID-19 vaccine encoding the RBD of the SARS-CoV-2 delta variant. The vaccine exhibits high efficacy in inducing anti-delta RBD neutralizing antibodies, which correlates significantly with the level of protection observed against severe COVID-19 in immunized mice.
As mentioned by the scientists, the mRNA-based nanoparticle-formulated vaccine developed in the study could be used as a flexible platform for rapidly developing effective vaccines against future variants of concern.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Brandys P. 2022. A mRNA vaccine encoding for a RBD 60-mer nanoparticle elicits neutralizing antibodies and protective immunity against the SARS-CoV-2 delta variant in transgenic K18-hACE2 mice. BioRxiv. https://www.biorxiv.org/content/10.1101/2022.03.21.485224v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Arginine, Assay, CCL5, Cell, Cell Proliferation, Chemokines, Coronavirus, Coronavirus Disease COVID-19, Cytokines, DNA, Efficacy, Enzyme, Immune Response, immunity, Immunization, Mortality, Nanoparticle, Omicron, Pandemic, Proliferation, Protamine, Protein, Public Health, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Transgenic, Vaccine, Virus

Written by
Dr. Sanchari Sinha Dutta
Dr. Sanchari Sinha Dutta is a science communicator who believes in spreading the power of science in every corner of the world. She has a Bachelor of Science (B.Sc.) degree and a Master's of Science (M.Sc.) in biology and human physiology. Following her Master's degree, Sanchari went on to study a Ph.D. in human physiology. She has authored more than 10 original research articles, all of which have been published in world renowned international journals.
Source: Read Full Article
