A new research paper was published in Oncotarget on July 28, 2022, entitled, “Chemoradiation-induced alteration of programmed death-ligand 1, CD8+ tumor-infiltrating lymphocytes and mucin expression in rectal cancer.”
DNA damage and resulting neoantigen formation is considered a mechanism for synergy between radiotherapy and PD-1/PD-L1 pathway inhibition to induce antitumor immune response.
In a new research study, by Marina Baretti, Qingfeng Zhu, Wei Fu, Jeffrey Meyer, Hao Wang, Robert A. Anders, and Nilofer S. Azad from Johns Hopkins University School of Medicine, researchers investigated neoadjuvant chemoradiotherapy (nCRT)-induced changes in CD8+ tumor infiltrating lymphocyte, PD-L1 and mucin expression in rectal cancer patients.
“The synergistic effects of CRT and PD-1/PD-L1 immunotherapy has been supported by several retrospective analyses in different cancer types, including esophageal cancer, bladder cancer, and lung cancer also support[ed] [14, 21, 22]. However, the role of nCRT to interact synergistically with immune checkpoint inhibitor treatment to improve tumor control in rectal cancer remains uncertain.”
The aim of this study was to evaluate nCRT-induced alterations in the TME [tumor microenvironment] of post-CRT resected specimens of rectal cancer, with a particular focus on PD-L1 expression and the density of CD8+ tumor-infiltrating lymphocytes (TILs). Tumor samples of rectal adenocarcinoma patients undergoing resection between 2008-2014 with (n = 62) or without (n = 17) nCRT treatment were collected.
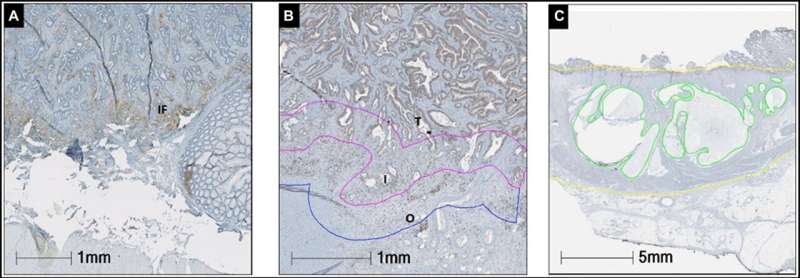
Sections were stained with CD8 and PD-L1 antibodies for immunohistochemistry. The prevalence of CD8+ cells was recorded in the tumor, interface tumor and background rectal side. Image analysis was used to determine the density of CD8+ lymphocytes. The percentage of PD-L1 expression was manually counted in tumor cells (TC), tumor stroma (TS) and the invasive front (IF). Mucin expression was determined as the percentage of the mucin area in the whole tumor area.
PD-L1 expression on TCs was identified in 7.6% (6/79) of nCRT specimens (p = 0.33) and in none of the non-nCRT patients. Median densities of CD8+ infiltrating T lymphocytes did not differ significantly between the two groups. Higher neutrophil to lymphocytes ratio (NLR) after nCRT was associated with worse outcome (HR = 1.04, 95% CI = 1.00–1.08). Mucin expression was significantly higher in the nCRT cohort (p = 0.02).
“These findings suggest a novel way through which radiation therapy can impact the immune TME towards a more immunosuppressive phenotype.”
nCRT exposure was associated with a non-significant difference in PD-L1 expression in rectal adenocarcinoma patients, possibly due to sample size limitations. Further mechanistic investigations and comprehensive immune analysis are needed to understand nCRT-induced immunologic shift in rectal cancer and to expand the applicability of checkpoint inhibitors in this setting.
“We showed that nCRT exposure was associated with a higher PD-L1 expression in tumor cells as compared to non-nCRT cases.”
In conclusion, the study provides further data on the immunologic impact of nCRT in rectal cancer. The researchers evaluated the effect of nCRT on CD8+TILsPDL-1 expression by spatial localization as well as on mucin expression, and their clinical implications in rectal cancer patients, comparing data from non-CRT cases. Matched pre- and postsurgical specimen analysis with further mechanistic investigations are needed in order to better evaluate the immune milieu of rectal cancer and to expand the applicability of checkpoint inhibitors in this setting.
Source: Read Full Article
