New orthopedic implant devices that promote faster healing and ultimately reduce the strain on the NHS could be available in the future thanks to new research by Loughborough University engineering experts that reveals which structures best promote bone healing.
The study, led by Dr. Carmen Torres-Sanchez, a Reader in Multifunctional Materials Manufacturing, tested implant designs currently in use and compared them to novel designs to better understand the structures bone-building cells favor.
Dr. Torres-Sanchez and her team of researchers found that the cells are sensitive to ‘topology’—the way in which structures are arranged in a design—and this can be exploited to help tissue heal faster.
The new paper, published in the Advanced Engineering Materials journal, even shows that the researchers were able to accelerate bone healing by making design tweaks.
Dr. Torres-Sanchez hopes the study findings will “see clinical application in the very near future to help trauma and bone cancer patients.”
The paper has also been included in a special series titled “Women in Engineering Materials,” praising its practical significance.
Implant design and previous studies
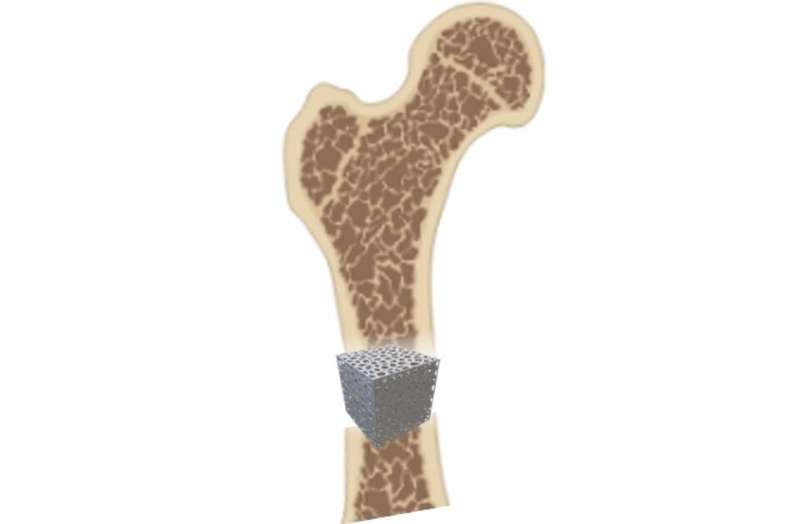
Orthopedic implants are medical devices that are used to replace missing joints or bone sections, or to support a damaged or diseased bone.
In the body, bones comprise voids and pores, which help give bone its biological and mechanical properties.
Implants look to mimic this porous structure in a bid to promote faster healing and integration of the implant in the body and replicate the mechanical properties of bone, including its ability to withstand forces generated by movement.
Two new types of designs were used in Dr. Torres-Sanchez’s study: triply periodic minimal surface (TPMS) and trabecular-like structures.
The study is one of the very few worldwide that assesses how design topology impacts biological and mechanical performance concurrently.

Study methods
Dr. Torres-Sanchez and team, in collaboration with industrial partners Alloyed Ltd and Core Specialists Ltd, tested the mechanical properties of TPMS and trabecular-like structures by 3D printing cubes—referred to as ‘scaffolds’—using a biocompatible material such as titanium.
The mechanical properties of the scaffolds were tested by applying forces that replicate the physiological loads that implants would be subjected to in the body, to find out whether the new designs could withstand them and at which point they would fail.
The biological performance of the designs was assessed by adding pre-osteoblasts—precursor cells to osteoblasts (bone-building cells) – to the inside the scaffolds to see if the cells could evolve into mineral matter, which forms bone.
Findings
The researchers found that cells prefer the more random distribution of porosity, such as that seen in trabecular scaffolds, as they seem to ‘identify them as home’ when pore structure is not organized.
The researchers were able to adjust the design of the ‘house’ where cells live to accelerate the formation of mineral matter.
Of the importance of the study, Dr. Torres-Sanchez commented: “Long-lasting successful implants, those that promote faster healing, without setbacks such as loosening or infections, without having second surgeries, are a no brainer for the NHS, for the patient, for society.
“The patient can go back to doing their normal life sooner, relieving the burden on hospitals, physiotherapy, carers, and contributing to a healthier, happier, more active life.
“We engineers can contribute to that by providing designs and scaffolds that promote healing and help speed up the patient recovery, including supporting mental health.
“We are continuing to research on fine-tuning the designs, so we can find subsequent evolutions of these multifunctional scaffolds that are even more appealing to the cells.”
Source: Read Full Article
