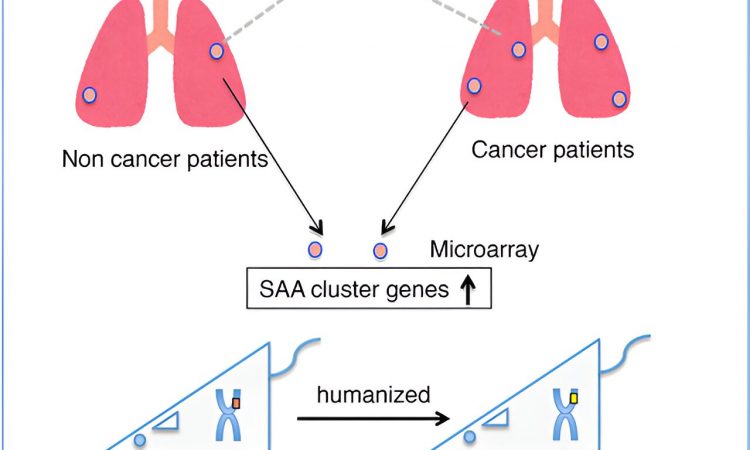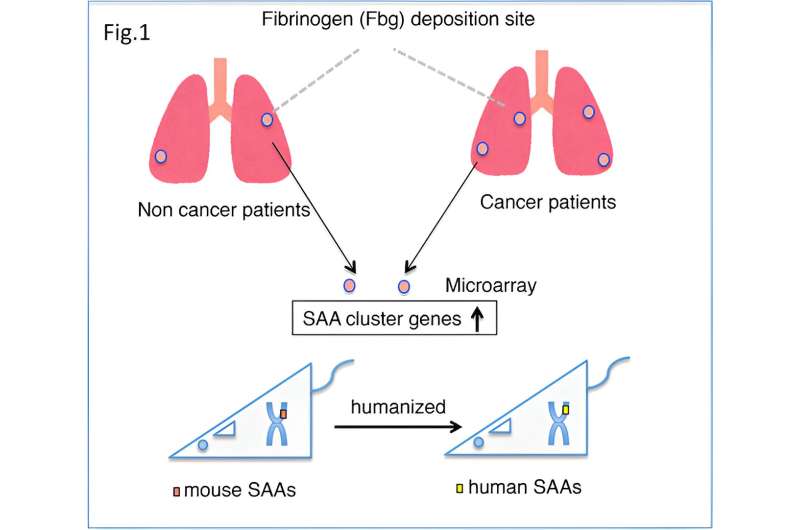
Cancer metastasis is the process by which cancerous cells spread from the primary tumor site to other regions of the body. Despite advancements in cancer research, the factors driving the selective spread of cancer to specific body regions remain largely unknown.
A study published in the journal Nature Communications on August 24, 2023, by researchers from Shinshu University School of Medicine, Japan, identifies an underlying mechanism.
“We indicate the possibility to detect the prediction site of metastasis by demonstrating that post-translational citrullination of fibrinogen creates a metastatic niche in the vulnerable spots. Pulmonary endothelial cells mediate the citrullination of fibrinogen, changing its conformation, surface charge, and binding properties with serum amyloid A proteins or SAAs, to make it a host tissue-derived metastatic pathogen,” explains Professor Sachie Hiratsuka from the Institute for Biomedical Sciences, Shinshu University School of Medicine, who led the study.
Citrullination is the process by which the amino acid arginine in a protein is changed into the amino acid citrulline.
In this study, the researchers have focused on this specific modification occurring in the protein fibrinogen, which contains one or more carbohydrate chains and plays a key role in the process of blood clotting.
Previous research carried out by Prof. Hiratsuka and colleagues had established a direct connection between discretely scattered fibrinogen deposits in the blood vessels of the lungs and higher odds of cancer metastasis, based on the data obtained from mouse models and patient samples.
To further delineate the mechanism of lung cancer metastasis, the team decided to closely examine the biochemical modifications that fibrinogen undergoes after it is synthesized in the body, i.e., post-translationally. First, they obtained autopsied lung tissue samples from patients with or without cancer.
After staining with dye, it became evident that the lung tissues of patients with cancer showed significantly more fibrinogen-positive areas than those obtained from patients who had no cancer at the time of death. Subsequent analysis revealed that the serum amyloid A (SAA) gene—a known pre-metastatic gene in mice—had a 5-fold higher expression in patients with cancer than in patients without cancer.
Armed with this knowledge, the team then generated humanized SAAs mice—rodents that are genetically programmed to produce a compatible human version of the SAA protein. As expected, lung tumor metastasis was more severe in the humanized SAA cluster mice than in their counterparts that lacked the SAA cluster genes.
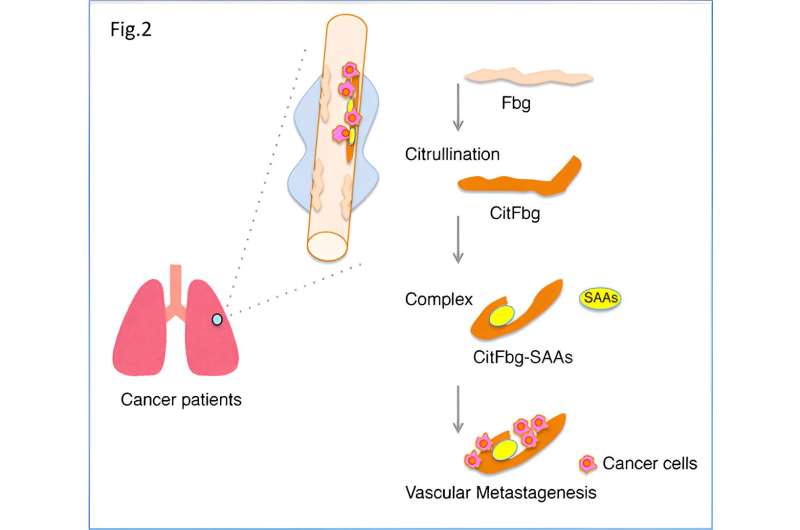
Next, the research team studied the interactions between the SAA3 protein and fibrinogen in mouse lungs harboring tumors. During the course of experiments, it became clear that SAAs mediated fibrinogen citrullination, changing the standard amino acid arginine into the non-standard citrulline.
Further experiments revealed that the SAAs-mediated citrullination of fibrinogen was causing lung tumor metastasis in the mouse model.
Finally, it became evident that a protein complex harboring citrullinated fibrinogen and SAAs (“SAAs-CitFbg”) was recruiting cancer cells and promoting lung tumor metastasis in the murine model. Based on these results, the team was able to successfully design a novel CitFbg peptide to counteract metastasis in the mouse model.
“A CitFbg peptide works as a competitive inhibitor to block the homing of metastatic cells into the SAAs-CitFbg sites. The potential metastatic sites in the lungs of patients were clearly visualized by our specific antibody for CitFbg,” adds Sachie Hiratsuka.
In summary, the study shows that CitFbg deposition is associated with an increased risk of metastasis in patients with cancer and that a citrullinated peptide can effectively counter this in a mouse model.
Prediction of cancer metastasis sites offers the opportunity for early-stage interventions, thus mitigating metastatic progression and life-threatening risks associated with cancer. Although clinical trials are warranted, the study nevertheless paves the way for the future development of novel anti-metastatic drugs.
These findings add substantial value to cancer research and may have important implications in the prevention and treatment of cancer.
More information:
Yibing Han et al, Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis, Nature Communications (2023). DOI: 10.1038/s41467-023-40371-1
Journal information:
Nature Communications
Source: Read Full Article