A recent review in the International Journal of Biological Macromolecules provides a novel overview of 'virosome'-based nanovaccine production, its properties, and its application in viral diseases, emphasizing its importance in SARS-CoV-2 vaccine discovery.
Virosome is a lipidic nanomaterial and is biomimetic. It can act as an immunogen, epitope-displaying nanocarrier, or delivery nanoplatform.
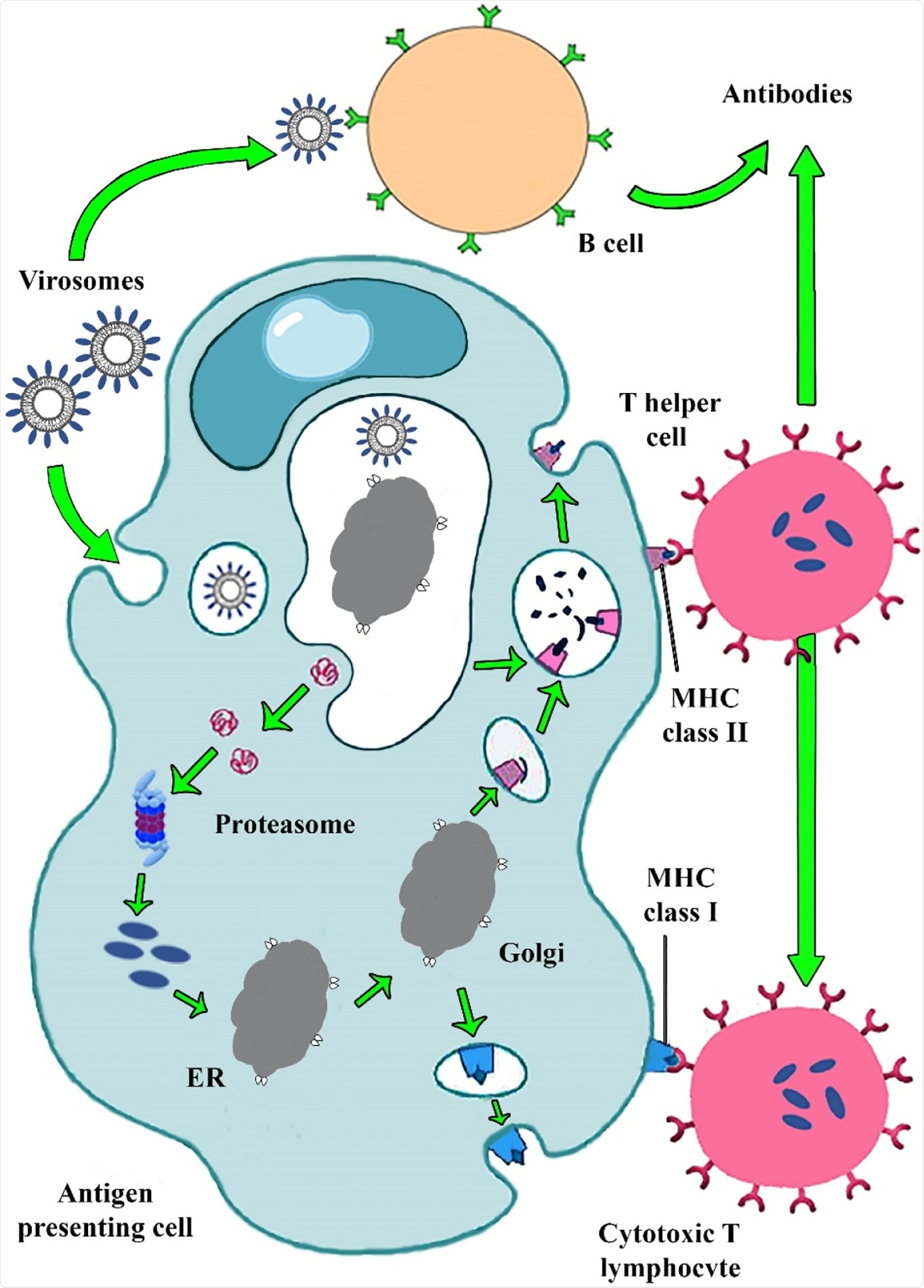
Virosome mimics the enveloped virus fusion pathway to evoke potent humoral and cellular immune responses."
It is an FDA-approved nanocarrier for pharmaceutical approaches, with promising bioinspiration and biomimetic potency against viral infections. The virosomes mimic the native morphology of the virus by incorporating different types of antigen epitopes. It thus has the potential to elicit strong immune responses in the host like a vaccine.
While vaccines protect us against malignancies and a wide range of contagious diseases, or even drug dependencies, conventional ones include weak cell-mediated immune responses, safety alarm of viral infections in vaccinated individuals, a requirement of booster doses, and the emergence of immunogenicity.
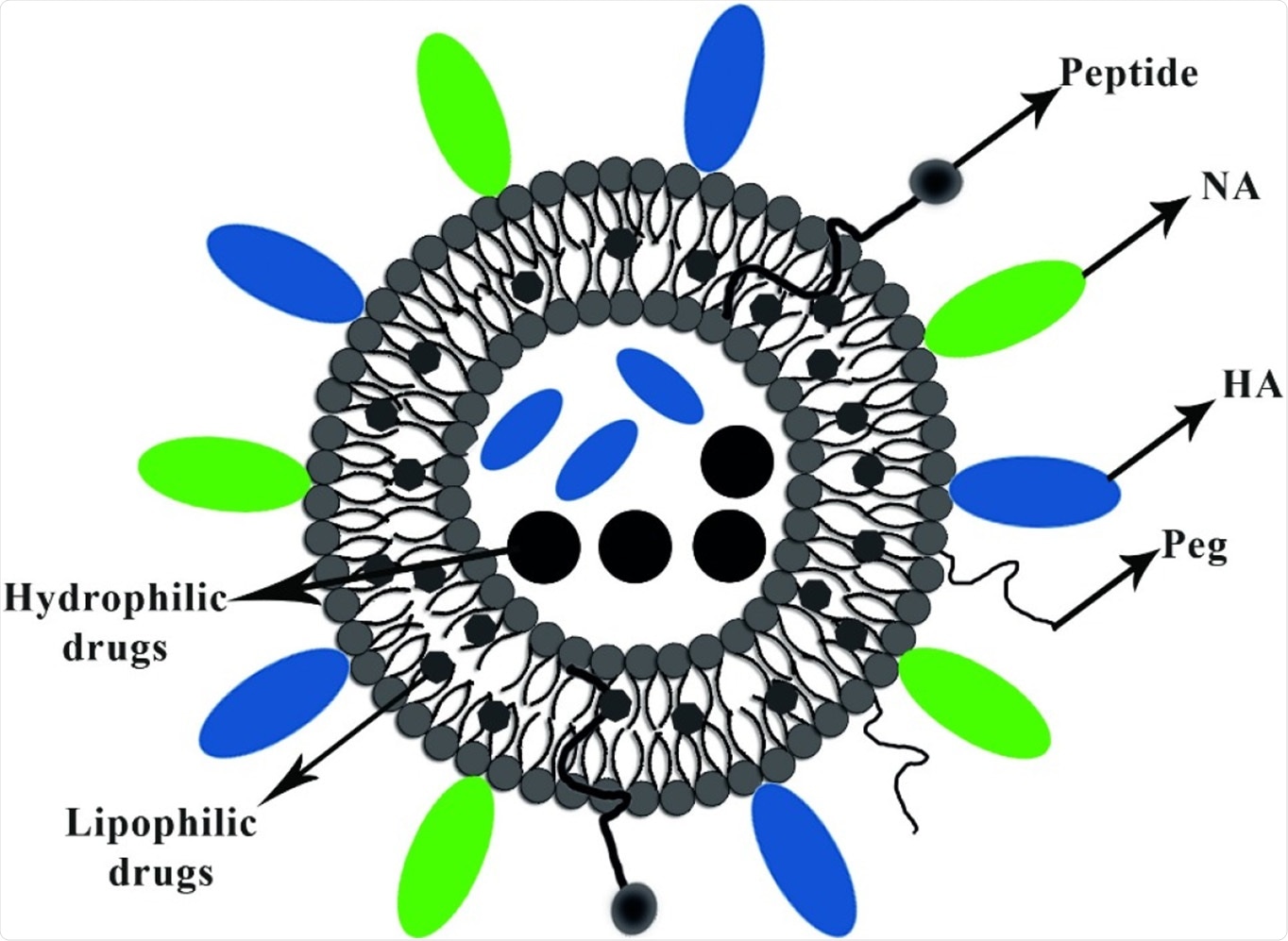
To address these limitations, nanomedicine comes to the rescue, contributing significantly to vaccine production. Nanocarriers utilized in vaccine formulations can be composed of lipids, proteins, metals, polymers, and other organic elements. Virosomes are nanocarriers – spherical, unilamellar vesicles (60–200 nm) with structural similarity to a phospholipid enveloped virus whose nucleocapsid is eliminated.
The virosome-based vaccine induces potent humoral and cellular immune responses critical in confronting life-threatening viral diseases. While RNA viruses mutate at high rates, virosomes can be versatile carriers that could develop multi-antigen epitopes against all strains of RNA viruses.
Potency and mechanisms of virosome-based vaccines actions is affected by several factors, such as chemical compounds, particle size distribution and homogeneity, charge, morphology, and location of antigens and/or adjuvants in a carrier structure and, finally, the route of administration."
Because virosomes cannot replicate but only incorporate into the host genome, this nanovaccine provides a new horizon in vaccine development and eliminates medical complications and concerns about next-generation products.
In the virosomes delivery system, the epitopes of antigens are adsorbed via hydrophobic domains or lipid linker on their surface and also inside, further integrating into the phospholipid bilayer. Viral glycoproteins epitopes are either on the virosome surface (peviprotm) or located in the hallow membrane vesicles (pevitertm).
The reviewers also discussed the possible ways of carrying the drugs; hydrophilic drugs are located in hollow vesicles, while hydrophobic agents mix with bilayer phospholipids. The surface modification of virosomes is done using hydrophilic polymers, such as polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), to increase their circulation time.
Choices with the targeting moieties (such as the ligand, antibody, and peptide) on the virosome structure provide opportunities to reach subcellular levels of tumor cells, respiratory and immune system cell components.
To date, several vaccine adjuvants based on virosome formulations have been commercialized against widespread and life-threatening infections, including influenza (Inflexal® V) and hepatitis A (Epaxal®)."
In the review, they offered their perspectives on virosomes as a biodegradable, biocompatible, nontoxic biomolecule with a high safety profile in vaccine development.
Other advantages of using the virosome include use of diverse administration routes and can also be combined with other adjuvants. The reviewers listed virosome-based formulations that are launched into the market, or in the preclinical and clinical stage. The reviewers reported that various types of virosome-based vaccines had been approved in over 45 countries, and more than 10 million patients have been immunized to date. They are also applicable for infants and older adults.
One of the major drawbacks of using a virosome is the rapid disintegration of virosome formulations. By stability optimization, thermostable virosome-based vaccines can be developed and administered via the mucosal route.
This is a bioinspiration compound that could revolutionize vaccination patterns in developing countries, the reviewers suggest. They also provided the classification of nanovaccines – polymeric nanovaccines, self-assembling peptides and protein vaccines, VLPs or viral structural proteins, inorganic nanovaccines, liposomal vaccines – widely used for viral infections in the clinic.
Virosome is thus an auspicious vehicle to develop vigorous nanovaccines. The preparation method is simple and possesses versatile delivery routes. Currently, many virosome products are already approved by authorities in different countries.
The researchers recommend that special efforts be put into virosome-based viral vaccine improvements, such as the Transvac 2, a new virosomal vaccine designed against SARS-CoV-2 viruses. Transvac 2 is in phase ΙΙ clinical trials. It is an innovative compound expected to be helpful in the recent viral pandemic.
In this review, the authors addressed important questions on the nature, preparation and popularity of the virosomes, highlighting their significance in the wake of COVID-19.
- Asadi, Khatereh and Ahmad Gholami. (2021) Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: A review. International Journal of Biological Macromolecules. https://doi.org/10.1016/j.ijbiomac.2021.04.005, https://www.sciencedirect.com/science/article/pii/S0141813021007601
Posted in: Medical Science News | Medical Research News | Miscellaneous News | Disease/Infection News | Healthcare News
Tags: Antibody, Antigen, Cell, Compound, Drugs, Genome, Hepatitis A, Immune System, Influenza, Ligand, Lipids, Macromolecules, Morphology, Nanomedicine, Pandemic, Particle Size, Pathogen, Peptides, pH, Polymers, Preclinical, Protein, Respiratory, RNA, SARS, SARS-CoV-2, Tumor, Vaccine, Virus

Written by
Dr. Ramya Dwivedi
Ramya has a Ph.D. in Biotechnology from the National Chemical Laboratories (CSIR-NCL), in Pune. Her work consisted of functionalizing nanoparticles with different molecules of biological interest, studying the reaction system and establishing useful applications.
Source: Read Full Article
