An open-access article in ARRS’ American Journal of Roentgenology (AJR) describes the clinical and imaging features of axillary adenopathy detected during screening or diagnostic breast imaging after recent coronavirus disease (COVID-19) vaccination to inform the development of follow-up recommendations.
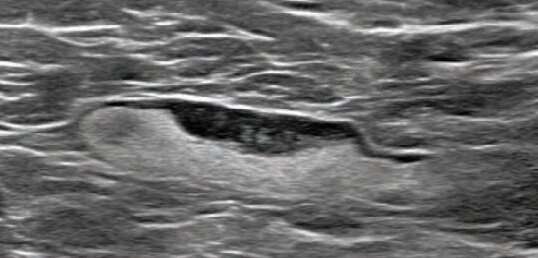
Shabnam Mortazavi of the University of California at Los Angeles reviewed electronic medical records to identify women with post-COVID-19 vaccination adenopathy found from December 2020 to February 2021. For mammography, Mortazavi considered a node abnormal when its size, shape, or density was deemed disproportionate to other axillary nodes (ipsilateral or contralateral). On ultrasound, she deemed a node abnormal based on subjective assessment for cortical abnormalities, including focal or diffuse thickening greater than 3 mm, as well as nodal prominence compared to the contralateral axilla (when available). For MRI, Mortazavi considered a node abnormal when asymmetric in size and/or number to the contralateral axilla.
Twenty-three women exhibited axillary adenopathy ipsilateral to the vaccinated arm on screening or diagnostic breast imaging, and according to Mortazavi, “13% were symptomatic (axillary lump with possible tenderness).” Meanwhile, the adenopathy was detected incidentally on screening breast imaging in 43% (mammography, 5; ultrasound, 2; both mammography and ultrasound, 1; high-risk screening MRI, 2) and on diagnostic imaging for other reasons in 43% (BI-RADS 3 follow-up for breast finding, 3; screening callback for other reason, 2; non-axillary breast pain or lump, 5). Noting that the median interval between the first vaccine dose and imaging showing the abnormal node was 9.5 days, Mortazavi’s results counted a total of 57% of women with one abnormal node. BI-RADS 2 was assigned in one woman, BI-RADS 3 in 21 (ultrasound in 4-24 weeks), and BI-RADS 4 in one.
“The largest sample to our knowledge of COVID-19 vaccine associated axillary adenopathy on imaging,” Mortazavi also wrote, “this study highlights axillary adenopathy ipsilateral to the vaccinated arm with Pfizer-BioNTech or Moderna COVID-19 vaccine as a potential reactive process with which radiologists must be familiar.” Ultimately, vaccination date and laterality are critical to optimize assessment and management of imaging-detected axillary adenopathy in women with otherwise normal breast imaging.
Source: Read Full Article
