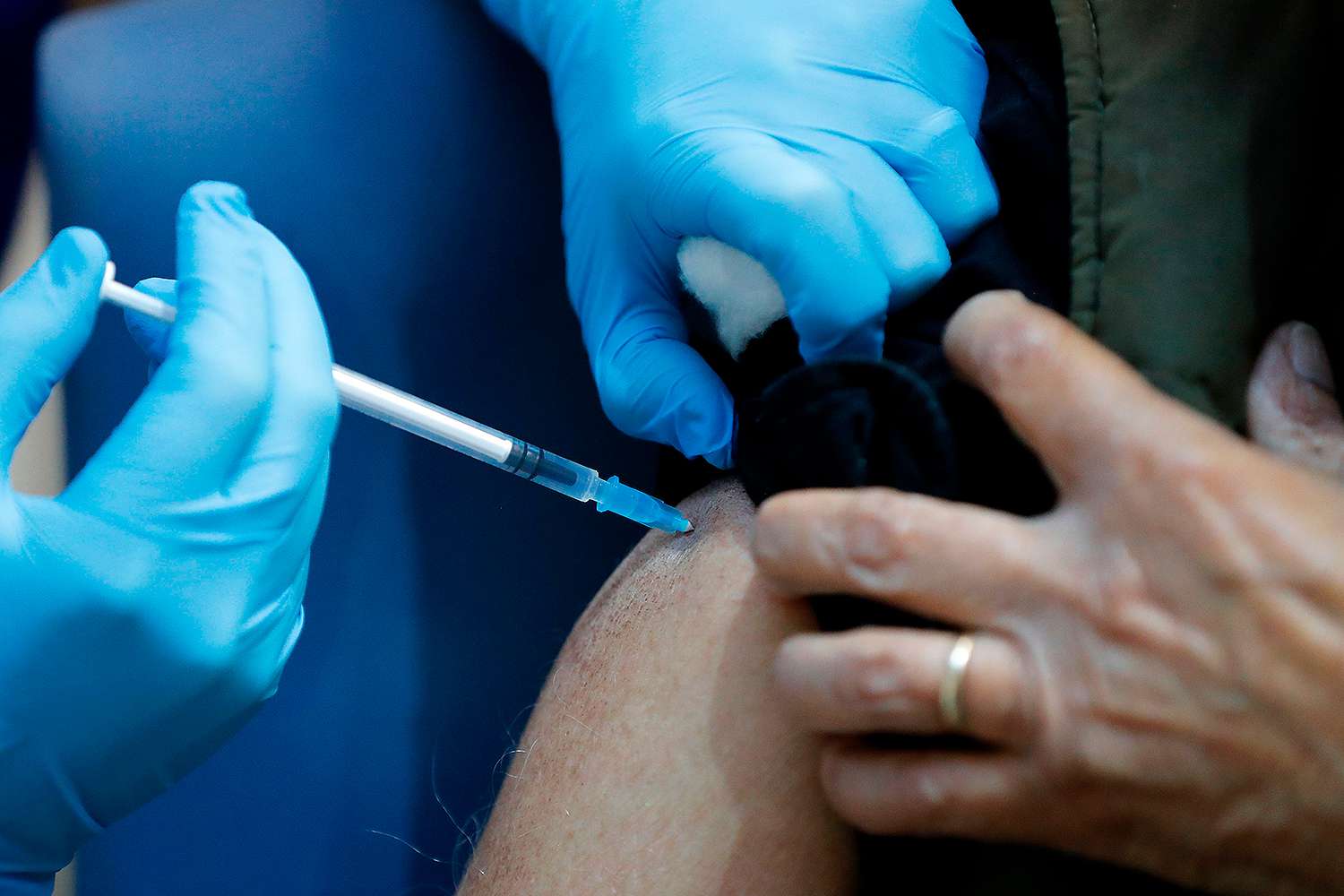
Americans could start getting vaccinated against COVID-19 as early as Monday, a senior health official said, as the Food and Drug Administration readies to approve a vaccine from Pfizer.
On Thursday, a panel of vaccine experts voted in favor of approving Pfizer’s COVID-19 vaccine for emergency use in the U.S. The decision now goes to the FDA, which is expected to fully approve the vaccine as soon as Saturday.
With that timeline in mind, Alex Azar, the U.S.’s secretary of Health and Human Services, said that the first inoculations could start early next week.
“We could see people getting vaccinated Monday, Tuesday of next week,” said on Good Morning America on Friday.
The FDA’s vaccine advisory panel voted 17 to 4 on Thursday in favor of authorizing the vaccine for use in Americans 16 years old and up, with one member abstaining. In the FDA’s scientific review, released Tuesday, they found Pfizer’s two-dose vaccine to be safe and effective, with the first shot providing more than 50 percent immunity and the second bringing immunity up to just under 100 percent.
Within 24 hours after the vaccine is officially approved, a first shipment of 6.4 million doses will go out from Pfizer’s warehouses to locations across the country, The New York Times reported.
Those first doses should go to frontline health care workers and residents and staff of long-term nursing homes, the Centers for Disease Control advised last week.
“With the high efficacy and good safety profile shown for our vaccine, and the pandemic essentially out of control, vaccine introduction is an urgent need,” Kathrin Jansen, a senior vice president and the head of vaccine research and development at Pfizer, said at the meeting.
Pfizer’s vaccine has already been approved in the United Kingdom and Canada. The U.K. started mass distribution of the vaccine on Tuesday, with the first shot going to a 90-year-old grandmother in England.
The FDA is expected to grant another vaccine, from Moderna, emergency use approval. Clinical trials showed that their vaccine is also highly effective, 94.5 percent, at protecting against COVID-19.
A COVID-19 vaccine is urgently needed in the U.S., with cases raging out of control. More than 223,570 Americans tested positive for the virus on Thursday, and at least 2,923 died from COVID-19, the second-highest daily death toll of the entire pandemic. Currently, 107,258 Americans are hospitalized with COVID-19, according to the COVID Tracking Project.
As of Dec. 11, more than 15,696,500 Americans have tested positive for COVID-19 since January, and at least 292,747 have died.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from the WHO and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article
