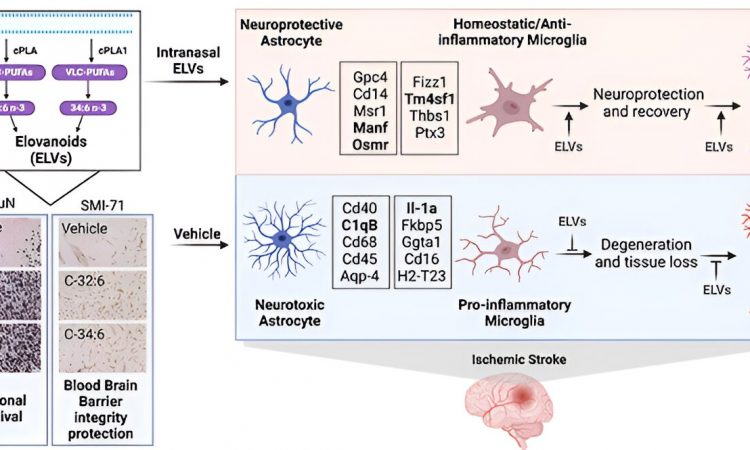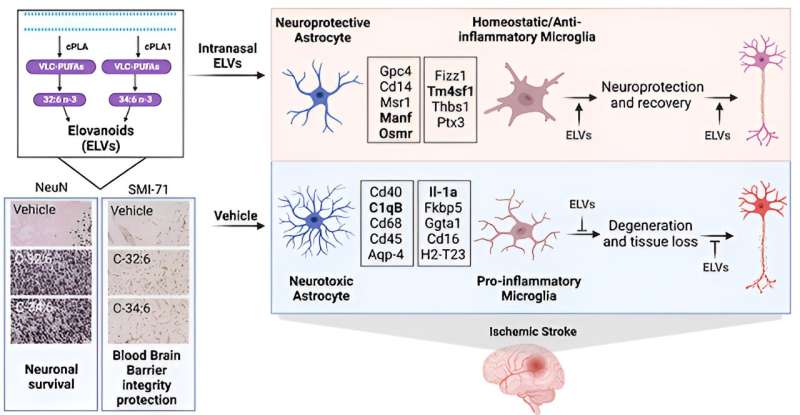
A study led by Nicolas Bazan, MD, Ph.D., Director of the LSU Health New Orleans Neuroscience Center, pinpointed which cells within specific areas of the brain are involved in post-stroke response and found that the delivery of precursors of Elovanoids, a class of molecules that synchronize cell-to-cell communication and neuroinflammation/immune activity in response to injury, improved neurological deficit in an experimental model of ischemic stroke.
The research team also identified cascades of gene responses as an integrated inflammatory signaling landscape, shedding light on potential new therapeutic avenues. Results are published in Scientific Reports.
Stroke treatment options are limited, particularly in the immediate aftermath of an event. Tissue plasminogen activator (tPA) by intravenous administration is a treatment for a limited number of patients after acute ischemic stroke with a narrow therapeutic window of 4.5 hours.
The recent EXTEND clinical trial evaluated the efficacy and safety of tPA administration beyond the standard 4.5-hour time window for acute ischemic stroke patients, which could potentially offer a longer treatment opportunity. As time elapses, the damage to the brain becomes progressively worse. Recent advances in endovascular intervention and enzymatic clot removal may facilitate reperfusion after ischemic stroke, yet there are still unmet needs to prevent and repair damage.
“Our paper reports the discovery that the administration of building blocks for precursors of Elovanoids through intranasal delivery can provide remarkable neuroprotection up to 7 days after stroke, based on a validated experimental model,” notes Dr. Bazan, who is also a Boyd Professor and the Ernest C. and Yvette C. Villere Chair for the Study of Retinal Degeneration at LSU Health New Orleans.
Despite efforts to identify neuroprotective mechanisms of damaging ischemic stroke cascade signaling, a void remains on an effective potential therapeutic. The present study defines neuroprotection by very long-chain polyunsaturated fatty acid (VLC-PUFA) Elovanoid (ELV) precursors C-32:6 and C-34:6.
The researchers found that free C-32:6 and C-34:6 reduced the activity of pro-inflammatory genes in the subcortex and cortex brain regions. Functional analysis also revealed upstream molecules related to detrimental signaling events in post-stroke acute and subacute phases.
“The mechanistic insights provided by our study demonstrate that through upstream effects, the free fatty acids act on genes which, when increased or decreased, aid in moving the sum of the signaling events toward recovery, regulating internal brain repair processes,” adds Dr. Bazan.
The study bridges a critical knowledge gap in stroke research. According to the Centers for Disease Control and Prevention, every 40 seconds, someone in the United States has a stroke. Every 3 minutes and 14 seconds, someone dies of a stroke. In 2021, 1 in 6 deaths from cardiovascular disease was due to stroke. Every year, more than 795,000 people in the United States have a stroke.
About 87% of all strokes are ischemic strokes, in which blood flow to the brain is blocked. Stroke-related costs in the United States came to nearly $56.5 billion between 2018 and 2019. This total includes the cost of health care services, medicines to treat stroke, and missed days of work.
“Further exploration of these findings for the development of targeted therapeutics holds promise for enhancing stroke outcomes and improving the lives of patients worldwide,” Bazan concludes.
More information:
Madigan M. Reid et al, Integrated inflammatory signaling landscape response after delivering Elovanoid free-fatty-acid precursors leading to experimental stroke neuroprotection, Scientific Reports (2023). DOI: 10.1038/s41598-023-42126-w
Journal information:
Scientific Reports
Source: Read Full Article