Analysis of data from more than 6,800 patients located across 16 countries has supported the need to increase the dose of the antimalarial drug, primaquine, in malaria endemic countries.
These results are detailed in two studies recently published in The Lancet Infectious Diseases, which reviewed the efficacy and safety of primaquine doses used to prevent recurrence of Plasmodium vivax (P. vivax) malaria.
Led by Menzies School of Health Research (Menzies) Senior Research Fellow, Dr. Rob Commons and The University of Melbourne Biostatistician, Dr. Megha Rajasekhar, this research is part of an international collaboration of malaria experts from the WorldWide Antimalarial Resistance Network (WWARN).
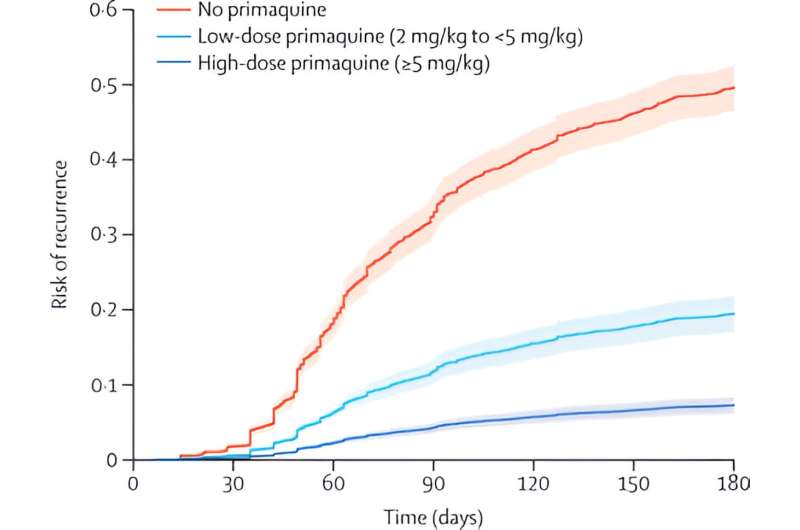
The study measuring efficacy found that increasing the dose of primaquine from 3.5mg/kg to 7mg/kg halved malaria relapses, with limited impact on gastrointestinal symptoms. The second study examined the safety of this dosing, with similar risks found between different primaquine dose regimens.
Primaquine is a medication used for more than 60 years to target malaria parasites in the liver and prevents infection from continuing. The results of the combined studies significantly increase understanding of the best primaquine dose to prevent malaria relapses.
P. vivax malaria affects more than 7 million people each year, mainly throughout the Americas, Africa and the Asia-Pacific. It puts 40% of the world’s population at risk of the infection. Once infected, P. vivax can hide in the liver for long periods of time before reappearing and causing a malaria relapse.
The findings around the safety of the higher primaquine regimen have the potential to pave the way for the widespread implementation of effective malaria treatment. Increasing the dose of primaquine could have a significant impact on reducing P. vivax malaria relapses, as well as deaths and malaria transmission.
Senior research fellow and lead co-author, Dr. Rob Commons, said, “These important papers highlight that we have been using primaquine at a suboptimal dose for several decades. Our findings show that using a higher dose of primaquine can reduce malaria relapses by over 50%. Most countries currently use a lower dose.
“Our results show the impact that increasing the dose could have on preventing people from getting recurrent malaria. This answers a question that the World Health Organization has highlighted as being a key research priority to resolve.”
University of Melbourne, Biostatistician and lead co-author, Dr. Megha Rajasekhar, said, “The second part of the study confirms that the increased dose is safe in patients at greatest risk of malaria relapses. We know that in patients with a genetic deficiency of the enzyme glucose-6-phosphate dehydrogenase—known as G6PD deficiency—the drug primaquine can cause hemolysis, or red blood cell destruction.
“Clinicians were worried that higher doses of the drug will cause side effects in people without this genetic deficiency, but our studies showed higher doses were safe for this group. New tests are now available to screen patients for G6PD deficiency, and this will allow higher doses to be prescribed safely, preventing malaria and speeding its elimination.”
WorldWide Antimalarial Resistance Network (WWARN) director, Professor Philippe Guérin, said, “The results of this study provide critical evidence supporting optimal dose of primaquine for radical cure of vivax malaria. This research is a great example of the malaria research community working together and demonstrates how data reuse can be at the forefront of generating new scientific knowledge.”
More information:
Robert J Commons et al, Effect of primaquine dose on the risk of recurrence in patients with uncomplicated Plasmodium vivax: a systematic review and individual patient data meta-analysis, The Lancet Infectious Diseases (2023). DOI: 10.1016/S1473-3099(23)00430-9
Megha Rajasekhar et al, Primaquine dose and the risk of haemolysis in patients with uncomplicated Plasmodium vivax malaria: a systematic review and individual patient data meta-analysis, The Lancet Infectious Diseases (2023). DOI: 10.1016/S1473-3099(23)00431-0
Journal information:
Lancet Infectious Diseases
Source: Read Full Article