In the face of growing antibiotic resistance among bacterial infections, scientists at The Hospital for Sick Children (SickKids) have delved into the molecular science behind treatment-resistant tuberculosis.
Tuberculosis (TB), an infection that primarily affects the lungs, is the most common cause of death by infectious disease worldwide. Although antibiotics have been the mainstay of treatment, the emergence of drug-resistant TB strains means that antibiotics used since the 1950s and 60s sometimes do not work and new treatments are needed.
In a study published in The EMBO Journal, a research team led by Dr. John Rubinstein, Senior Scientist in the Molecular Medicine program, and first-author Gautier Courbon, a Ph.D. Candidate in the Rubinstein Lab, examined how two new compounds attack the bacteria that cause TB, providing insights that may inform future drug therapies.
“New treatments for TB are frequently tested, but the mechanisms behind their success, or failure, are not always understood,” says Rubinstein. “By determining structures of candidate drugs bound to their targets and identifying how these compounds work on a molecular level, we hope to help inform future therapies.”
Exploring new compounds to reduce side effects
TB is caused by a type of bacteria called mycobacteria. Compared to most other bacteria, mycobacteria are naturally more treatment-resistant, in part due to their slow growth rate. Mycobacterial growth is reliant on oxygen to break down molecules from the host’s body for energy, a process known as aerobic respiration. This process uses a protein called adenosine triphosphate (ATP) synthase to convert the energy into a form that bacteria can use.
The current treatment for drug-resistant TB relies on a recently developed drug called bedaquiline (BDQ), which targets ATP synthase, effectively stopping the bacteria from producing the energy they need to survive.
Despite the success of BDQ in treating drug-resistant TB, resistant mycobacteria have started to emerge and its side effects can include liver toxicity and heart failure, prompting scientists to seek out effective treatments with fewer side effects.
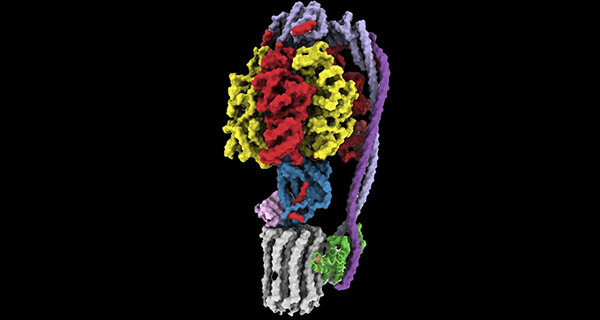
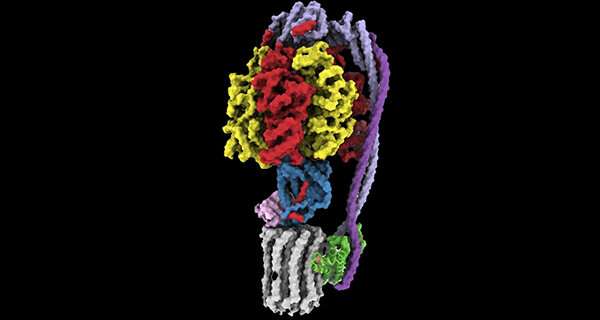
Led by Rubinstein, the research team used state-of-the-art imaging at the SickKids High-resolution high-throughput CryoEM core facility to examine two new compounds that target ATP synthase, called TBAJ-876 and SQ31f.
Unveiling new avenue to attack TB infections
The study showed why TBAJ-876, which is derived from BDQ and is currently undergoing clinical trials, binds to ATP synthase better than BDQ. The team also discovered that although the current form of SQ31f prevents mycobacteria from growing but does not kill them, it binds to ATP synthase at a previously unknown site, which could allow development of an entirely new class of antibiotic that works even for BDQ-resistant strains. The work builds on the Rubinstein group’s discovery of how BDQ binds ATP synthase, which they published in Nature in 2020.
“Unlike BDQ and TBAJ-876, SQ31f binds a previously unknown site on ATP synthase,” explains Rubinstein, who also holds a Canada Research Chair in Electron Cryomicroscopy. “This could represent an entirely new avenue to attack mycobacterial infections.”
In addition to informing the development of TBAJ-876 and SQ31f, these findings also offer fresh insights into the molecular mechanisms that make some ATP synthase inhibitors more effective at killing mycobacteria than others. By better understanding these mechanisms, the research could be used to support development of new therapeutic strategies in the future to combat TB and other mycobacterial infections, including those that affect patients with depressed immune systems or cystic fibrosis.
The development and testing of new drugs is a long process so it may be many months or years before these compounds are released for patients, but the research team is optimistic that their contributions will help inform future treatments for patients with TB.
More information:
Gautier M Courbon et al, Mechanism of mycobacterial ATP synthase inhibition by squaramides and second generation diarylquinolines, The EMBO Journal (2023). DOI: 10.15252/embj.2023113687
Journal information:
Nature
,
EMBO Journal
Source: Read Full Article