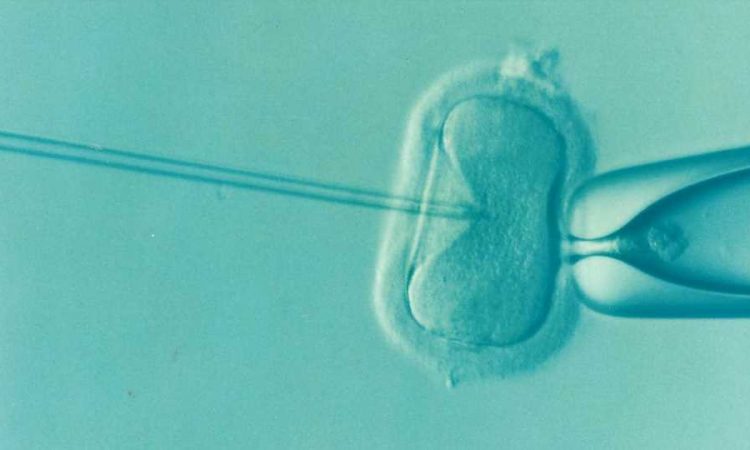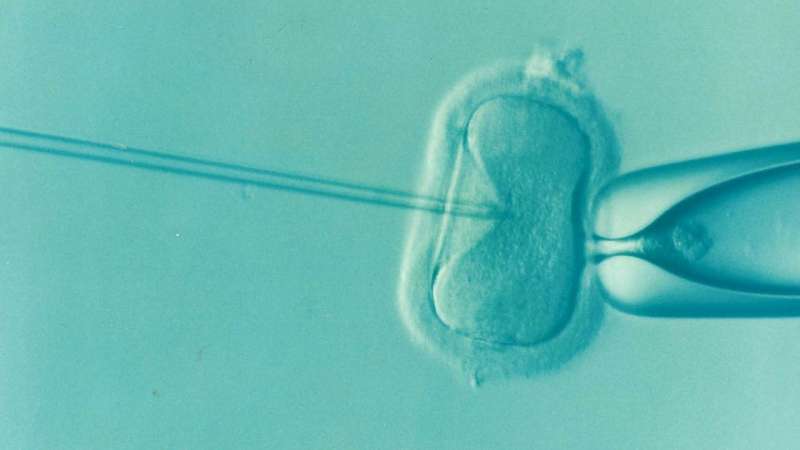
Although transgender and gender diverse young adults demonstrate a high interest in parenting, less than 20% of transgender and nonbinary individuals in the United States are parents.
Transgender and gender diverse people who wish to become parents face barriers including discrimination in the health care setting, access to high quality fertility care and high cost as fertility care is not typically covered by public or private insurers.
Beyond logistic barriers to receiving fertility treatment, the potential stress of halting gender-affirming hormones before treatment poses an additional challenge.
Organizations recommend doctors council patients who are beginning gender-affirming treatment about fertility preservation to avoid pausing hormones later.
However, freezing eggs or sperm is often cost prohibitive for an 18 or 19-year-old adult patient beginning gender-affirming hormones.
The current recommendation is for patients to stop taking gender-affirming hormones for up to a three month “washout period” before in vitro fertilization, commonly known as IVF, says Molly Moravek, M.D, M.P.H., MSCI, a reproductive endocrinologist at Michigan Medicine.
“It’s unfair that we are advising people to make life changing interventions by spending thousands of dollars to freeze eggs or halting testosterone for three months before IVF out of fear that testosterone treatment might affect their fertility,” said Moravek.
“We worry testosterone might affect their fertility outcome, but we don’t have any evidence for that claim.”
Investigating IVF treatments for transgender patients
To remedy this, Moravek led a University of Michigan team with Ariella Shikanov, Ph.D., and Vasantha Padmanabhan, Ph.D., to investigate how gender-affirming testosterone treatment affected IVF outcomes using a mouse model.
Researchers divided genetically identical female mice into four treatment groups: 1) control implant, 2) testosterone implant 3) control implant washout, 4) testosterone implant washout.
The first two groups underwent ovarian stimulation for egg collection with hormone implants in place while the washout groups had the hormone implant removed for two weeks before ovarian stimulation.
Eggs were then collected, fertilized and cultured in vitro for early embryonic stages.
The experiment was conducted twice with testosterone implants in place for six or 12 weeks to represent short and long-term testosterone exposure.
The researchers found that testosterone treatment decreased egg yield without affecting quality.
For the short-term testosterone treatment group with a two-week washout, IVF outcomes were fully recovered to the level of controls.
However, IVF outcomes were not fully recovered for the long-term testosterone treatment group.
“In mice, we’re seeing that you may get less eggs with testosterone treatment, but those eggs are fine and still create normal embryos that become live born offspring,” said Moravek.
Next steps
The researchers plan to investigate whether there is a medication that can clear out the eggs to hasten the washout period.
Moravek also plans to study multiple generations of mice to determine whether the offspring of gender-affirming testosterone treated parents experience any reduced fertility or metabolic effects.
As evidence builds, this information can help inform conversations between doctors and patients when deciding whether to halt gender-affirming testosterone treatment before IVF.
“Some patients are comfortable halting gender-affirming testosterone. For others, it can be very distressing,” said Moravek.
For the subset of patients who would be distressed by stopping gender-affirming testosterone, ovarian reserve—or the number of eggs remaining in the ovaries—can help direct the conversation, says Moravek.
The egg loss may not be a concern for a patient with a great ovarian reserve but for a patient with a low ovarian reserve, it will be important to discuss the pros and cons of continuing gender-affirming testosterone.
“We owe it to our patients to have data to back up decisions before we tell them to do these expensive and emotionally taxing procedures,” said Moravek.
The work is published in the American Journal of Obstetrics and Gynecology.
More information:
Amanda R. Schwartz et al, Impaired in vitro fertilization outcomes following testosterone treatment improve with washout in a mouse model of gender-affirming hormone treatment, American Journal of Obstetrics and Gynecology (2023). DOI: 10.1016/j.ajog.2023.07.013
Journal information:
American Journal of Obstetrics and Gynecology
Source: Read Full Article