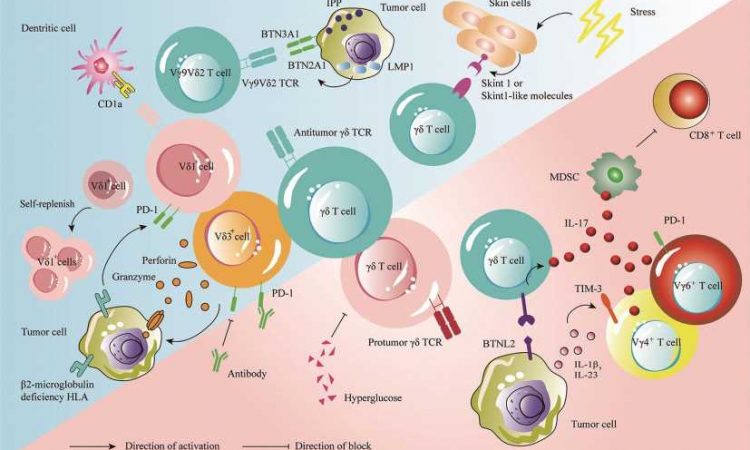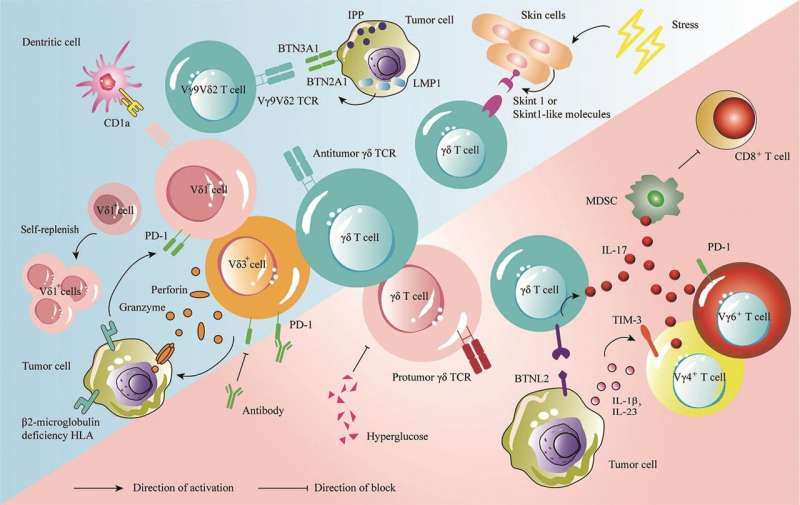
Gamma delta (γδ) T cells are a type of unique innate T lymphocyte that are considered to be among the best candidate cells for use in immunotherapy. However, owing to their rarity in the peripheral circulation and lymphoid organs, their precise role in regulating antitumor immune response remains more or less unclear.
Recent advances in tumor immunotherapy have, however, increased the focus of research on γδ T cells. Of particular interest is their strong cytotoxicity against a variety of tumor cells without major histocompatibility complex restriction.
Summarizing the recent developments in this field, a review article published in the Chinese Medical Journal now outlines the overall progress in research on γδ T cells in the year 2022.
The basic research and clinical application enlisted in this review strives to provide a theoretical basis for the development of γδ T cell-based clinical interventions in the near future. The review is authored by Wei He, Jianmin Zhang, and Hui Chen from the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences (CAMS). Their team has made dedicated efforts for the clinical transformation and application of γδ T cells for more than 20 years and is deeply involved in this field.
Prior studies have shown that mature γδ T cells are abundantly present in the skin as well as in the digestive, respiratory, and reproductive tracts. Various types of solid tumors are also likely to be found in these precise regions of the body. In addition, γδ T cells multiply easily and are well-suited for immunotherapy applications. However, details surrounding multiple other functionalities of γδ T cells (e.g., development, activation, and function) continue to emerge.
Moreover, current γδ T cell-based therapies are based on two main biochemical mechanisms. The first involves the selective amplification of γδ T cells in vivo with antibodies/bisphosphonate antigens. The second involves adoptive cell therapy—an immunotherapy-based treatment involving the collection/modification of immune cells (including γδ T cells) from a donor (self or a different individual) and their final introduction into the patient’s body.
In this review now, the authors report that multiple research groups have identified various hidden γδ T mechanisms in 2022. For instance, a landmark Nature Immunology study found that when mouse skin was exposed to common forms of stress (e.g., ultraviolet radiation or harsh chemicals), γδ T cells were able to detect changes in the cellular environment and help the skin recover.
This finding has clear implications in the area of skin cancer. Yet another study published in Nature journal revealed that γδ T cells play a key role in detecting and eliminating tumors that remained invisible to the immune system’s “conventional” defense mechanisms.
Furthermore, a study published in the Science journal showed that certain subpopulations of γδ T cells harbored both antitumor and protumor subtypes. Published evidence also shows that like many other immune cells, γδ T cells also exhibit a “split personality.”
For instance, certain subpopulations of murine γδ T cells, particularly those that secrete the pro-inflammatory protein “interleukin-17,” tend to suppress the immune response instead of activating it, thus offering “diplomatic immunity” to rogue tumors. Therefore, studying the underlying mechanisms of action becomes extremely necessary prior to clinical application.
Speaking about the research advances further, senior author Hui Chen, affiliated with the Changzhou Xitaihu Institute for Frontier Technology of Cell Therapy and Peking Union Medical College, says, “Tumor-infiltrating γδ T cells have been shown to be the immune cells that are most positively associated with patient survival in all cancers. When examining brain tumor samples from patients, γδ T cell infiltration was shown to be the strongest predictor of survival, while αβ T cell infiltration was negatively associated with survival.”
The authors further report that by the end of early 2023, a total of 43 clinical trials related to γδ T cell immunotherapy were registered. Further, multiple trials currently underway show promising results.
For instance, the interim results of a phase 1a/2b clinical study of a natural γδ T cell product demonstrated good safety and efficacy in treating acute myeloid leukemia—a type of cancer that affects the bone marrow and the blood.
The authors also delve into the γδ T cell-based immunotherapy strategies, including genetically modified chemotherapy resistant γδ T cell allotherapy, CAR- γδ T cell therapy, natural, unmodified γδ T cell allotherapy, and allogenic Vδ 1 γδ T cell therapy.
In summary, the review clarifies the extent to which latest research on γδ T cell-based immunotherapy has answered the longstanding questions and highlights the need for more extensive and in-depth mechanistic studies.
Specifically, the authors put forth the need to study the characteristics and functions of γδ T cells in humans, their underlying role in IL-17 production, and their interactions with other cells in vivo. These findings, in turn, are likely to pave the way for tumor-immunotherapy-based clinical applications of this key group of white blood cells in the near future.
More information:
Yueqi Zhao et al, γδ T cells: Major advances in basic and clinical research in tumor immunotherapy, Chinese Medical Journal (2023). DOI: 10.1097/CM9.0000000000002781
Journal information:
Nature
,
Nature Immunology
,
Science
Source: Read Full Article