Research conducted at the Centenary Institute has unveiled crucial insights into the underlying molecular mechanisms of breast cancer. The findings could lead to a more effective treatment for the disease, which claims the lives of more than 3,000 Australian women and men annually.
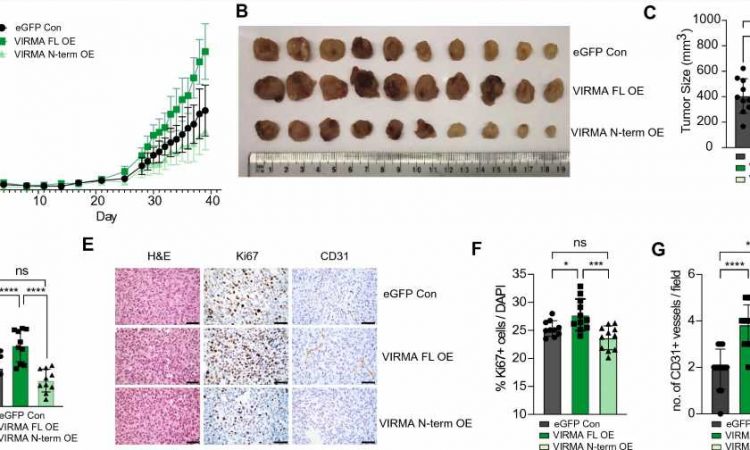
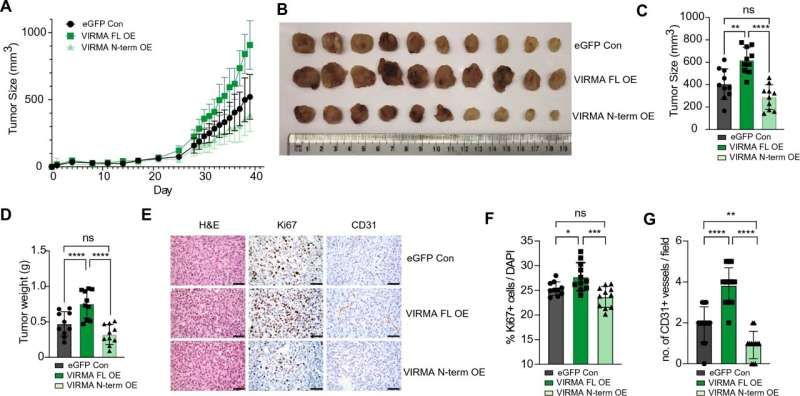
The study, involving mice, focused on the role of a protein known as VIRMA in breast tumor growth. Researchers found that abnormally high levels of VIRMA play a key role in promoting the development of breast cancer cells and are closely associated with poor survival outcomes.
Specifically, the study revealed that a distinct variant of VIRMA, localized within the cell nucleus, is amplified and overexpressed in 15%–20% of breast cancers. VIRMA was shown to drive the growth of breast cancer cells by influencing a chemical modification of RNA molecules.
Additionally, the research identified a specific RNA molecule called NEAT1, which interacts with VIRMA and that also promotes the growth of cancer cells.
Dr. Justin Wong, Head of the Epigenetics and RNA Biology Program at the Centenary Institute, and senior author of the study published in the journal Cellular and Molecular Life Science, notes that breast cancer cells with elevated levels of VIRMA exhibited heightened susceptibility to death under prolonged stressful conditions. This newfound vulnerability could pave the way for a novel therapeutic strategy to treat breast cancer.
“Based on our findings, we can repurpose certain therapeutic drugs that trigger stress response to target and eliminate breast cancer cells that have high levels of VIRMA. By targeting VIRMA-overexpressing cancer cells, the aim is to enhance stress response in cancer cells and ultimately force them to commit suicide,” said Dr. Wong.
He added, “Breast cancer remains a pressing public health concern and this research breakthrough provides renewed hope for the development of targeted and impactful treatments.”
More information:
Quintin Lee et al, Overexpression of VIRMA confers vulnerability to breast cancers via the m6A-dependent regulation of unfolded protein response, Cellular and Molecular Life Sciences (2023). DOI: 10.1007/s00018-023-04799-4
Journal information:
Cellular and Molecular Life Sciences
Source: Read Full Article