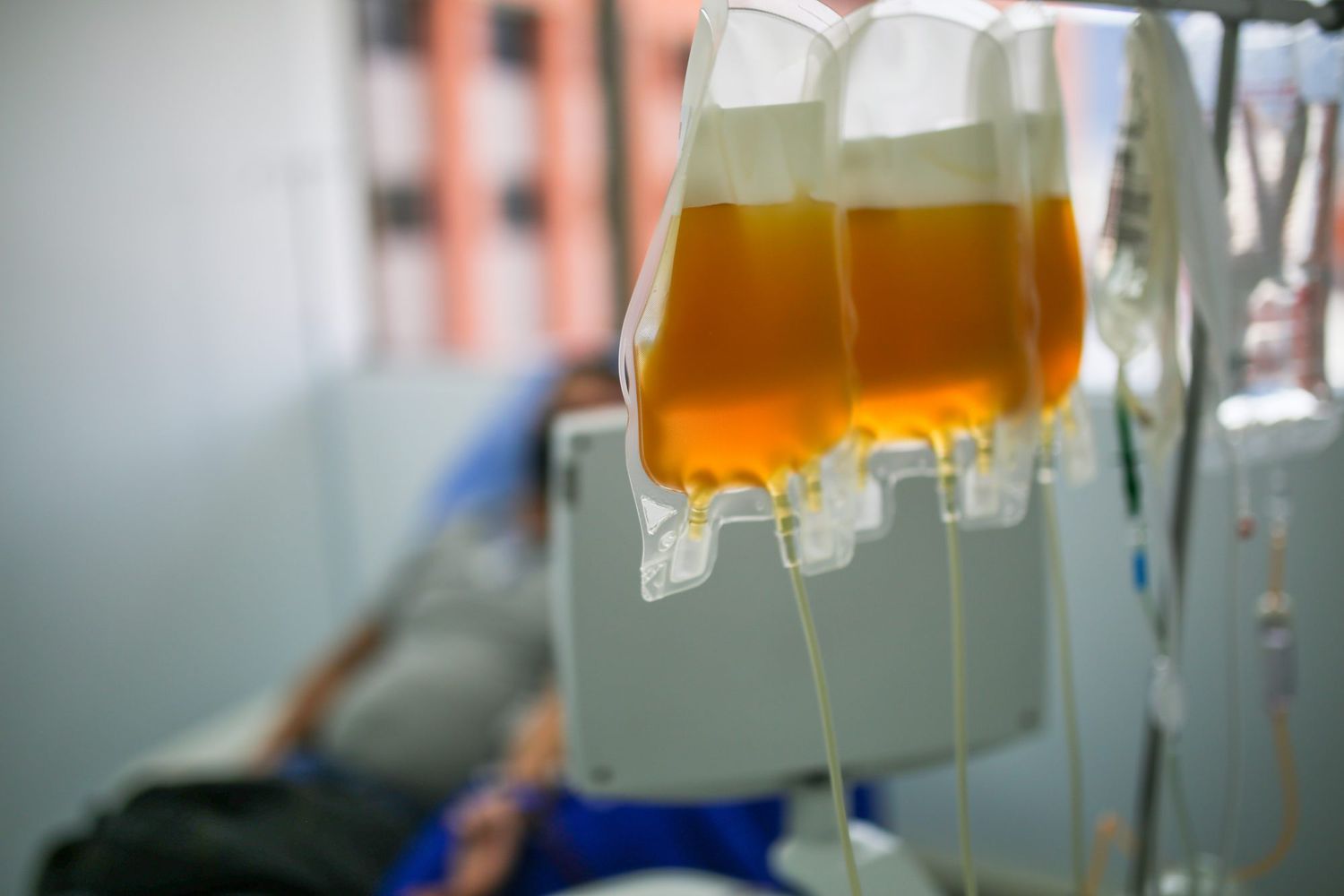
There is no evidence yet that convalescent plasma is an effective treatment for COVID-19, a panel at the National Institutes of Health said Tuesday — and it should not be used on current patients.
"There are currently no data from well-controlled, adequately powered randomized clinical trials that demonstrate the efficacy and safety of convalescent plasma for the treatment of COVID-19," the COVID-19 Treatment Guidelines Panel at NIH wrote, in a statement shared online.
The report comes just days after President Donald Trump — in conjunction with the Food and Drug Administration — called convalescent plasma a “breakthrough” treatment and gave emergency authorization for its use on COVID-19 patients in the U.S.
In contrast to Trump’s assertions, the NIH panel’s statement said that “there are insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19.”
“Convalescent plasma should not be considered standard of care for the treatment of patients with COVID-19,” they said.
The NIH based its recommendation on all available research on convalescent plasma, including the studies that led to the FDA’s emergency use authorization.
The FDA had cited a preliminary study on convalescent plasma from the Mayo Clinic, which found that COVID-19 patients who were sick enough to be hospitalized but did not need to be on a ventilator were slightly helped by the antibody-rich serum, which comes from the blood of recovered COVID-19 patients. However, the Mayo Clinic researchers did not find a significant improvement in death rates from using the plasma.
Around 72,000 COVID-19 patients in the U.S. have been treated with convalescent plasma throughout the pandemic, NBC News reported, but the effectiveness from those treatments are not clear.
The NIH panel said that “prospective, well-controlled, adequately powered randomized trials are needed to determine whether convalescent plasma is effective and safe for the treatment of COVID-19.”
The FDA’s decision to grant an emergency use authorization for convalescent plasma immediately drew criticism from health experts, including top coronavirus advisor Dr. Anthony Fauci. Some said the decision was politicized and made to please Trump. FDA Commissioner Stephen Hahn, who touted convalescent plasma along with Trump on Aug. 23, apologized one day later for overstating the benefits of the serum.
“I have been criticized for remarks I made Sunday night about the benefits of convalescent plasma. The criticism is entirely justified. What I should have said better is that the data show a relative risk reduction not an absolute risk reduction,” Hahn tweeted.
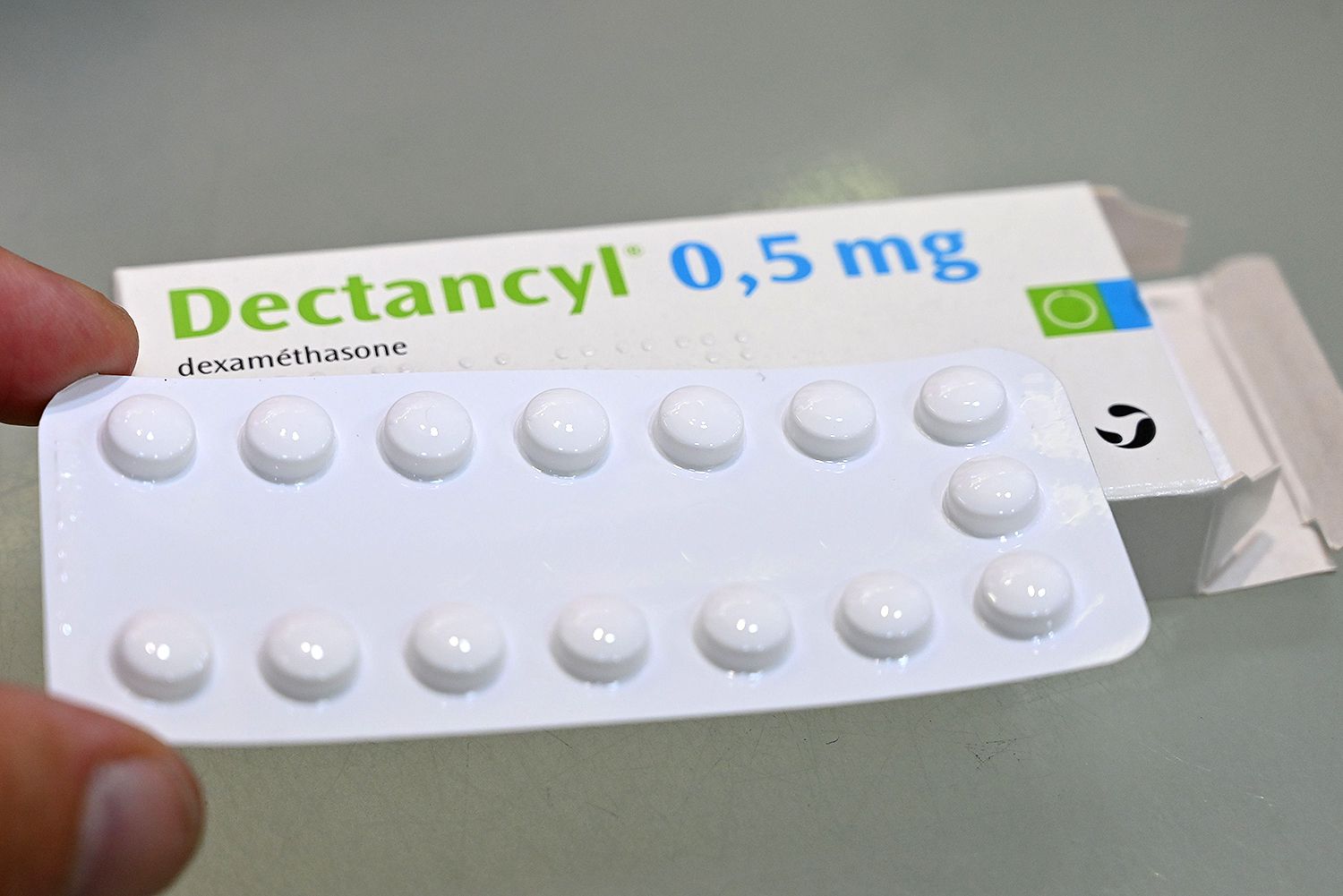
While the effectiveness of convalescent plasma is still in question, a cheap, widely-available steroid has been confirmed to help severely sick COVID-19 patients, according to four studies published Wednesday, The New York Times reported. The studies were the result of several international clinical trials testing the use of three steroids — dexamethasone, hydrocortisone and methylprednisolone — in more than 1,700 patients.
The steroids all helped to calm the body’s immune reaction to COVID-19 and lessen inflammation, swelling and pain. Dexamethasone was the most effective, leading to a 36 percent drop in deaths in 1,282 treated with the drug.
The World Health Organization said that based on this research, they strongly recommend using the steroids in severely sick COVID-19 patients.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from CDC, WHO, and local public health departments. To help provide doctors and nurses on the front lines with life-saving medical resources, donate to Direct Relief here.
Source: Read Full Article
