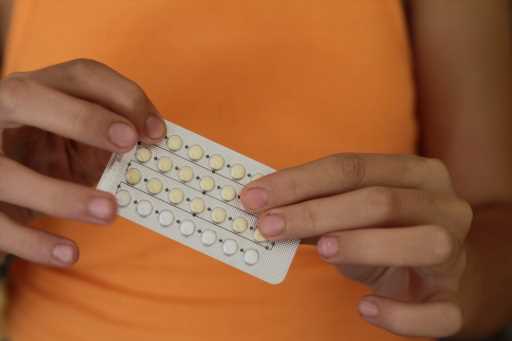
Today, the U.S. Food and Drug Administration (FDA) has officially approved Opill, a daily birth control pill, for over-the-counter sale.
Opill’s FDA approval means it’s the first over-the-counter birth control option that’s available in the U.S. without a doctor’s prescription. That could go a long way in eliminating barriers for people who need birth control but are uninsured, are unable to see a doctor, or are in a sexual health clinic or contraceptive desert.
The FDA statement noted that OTC birth control can make a huge difference in preventing the over 3 million unintended pregnancies in the U.S. each year. “Unintended pregnancies have been linked to negative maternal and perinatal outcomes, including reduced likelihood of receiving early prenatal care and increased risk of preterm delivery, with associated adverse neonatal, developmental and child health outcomes,” the statement read.
Like other progestin-only birth control pills, Opill must be taken at the same time every day and is not completely without risks, but that’s pretty standard. Spotting, nausea, and headaches are common side effects of almost any hormonal birth control and often are more prominent when you first start taking it.
When will Opill be available? CNN reported that it’ll be in pharmacies and drug stores by the beginning of 2024. The price is still to be determined, but there are efforts to make it as affordable and accesible as possible to anyone who needs over-the-counter birth control.
The FDA’s vote comes at a tenuous time for abortion access in America.

Image Credit: Getty Images Access to birth control is more vital than ever, especially for people who live in states with hostile abortion laws.
Last summer, Roe v. Wade — the landmark Supreme Court ruling that safeguarded access to abortions nationwide for nearly 50 years — was reversed. States are now able to ban or restrict abortions, and many already have.
Meanwhile, the Centers for Disease Control and Prevention (CDC) estimates that 45 percent of all U.S. pregnancies are unintended. That’s why the FDA advisers voted to approve Opill for OTC use. There are some concerns about people using the pill incorrectly, but ultimately, experts believe the pros of making it more accessible outweigh the cons.
Kate Curtis, an FDA adviser who works for the CDC, told CNN that she voted yes because Opill “has the potential to have a huge positive public health impact.”
What is Opill?

Image Credit: Getty Images/PhotoAlto Opill is a mini-pill, meaning it only uses the hormone progestin. Progestin-only birth control was approved by the FDA for pregnancy prevention in 1973 and has been used safely ever since. Like most hormonal birth control pills, it is taken daily at roughly the same time each day.
Daily birth control pills are just one form of contraception, and since they involve hormones, they are sometimes associated with less-than-desirable side effects. Non-hormonal options for pregnacy prevention include condoms, spermicidal gel, or copper IUDs.
Unsure which type of birth control is best for you? Talk to your healthcare provider, who can help you make the decision.
Source: Read Full Article