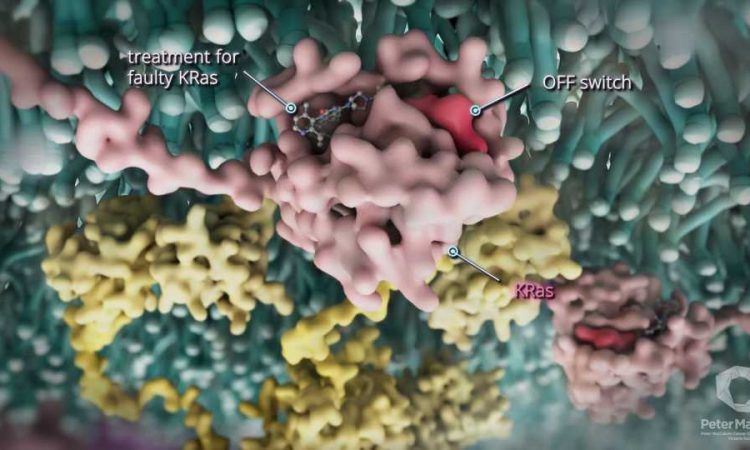
A new tablet treatment called divarasib has shown it is very effective in treating a challenging type of bowel (colorectal) cancer associated with the KRAS G12C mutation.
Research, published in Nature Medicine today (Dec. 5), has shown remarkable results in 62% of people with KRAS G12C mutated bowel cancer achieving a positive response to treatment when given divarasib in combination with another cancer treatment called cetuximab.
Professor Jayesh Desai, Medical Oncologist, Associate Director of Clinical Research and Head of Early Drug Development Trials at the Peter MacCallum Cancer Center, explained that these results are incredibly impressive. Although the KRAS G12C mutation only occurs in approximately 4% of patients with colorectal cancer, it is routinely tested for, so our ability to identify the right patients to offer such trial treatment to is straightforward.
“The median progression-free survival for patients in the study was just over eight months and the treatment was well tolerated with manageable side effects,” Professor Desai said.
“While this is not a head-to-head trial the response rates are better than what we have seen with other treatments that work on the KRAS G12C mutation pathway.
“We are very hopeful that this combination of divarasib with cetuximab will translate into better outcomes for our colorectal cancer patients,” he said.
Professor Desai emphasized the important role of the Parkville Cancer Clinical Trials Early Drug Development team in recruiting, supporting and managing patients through these challenging early phase clinical trials.
“Early drug development trials require a very experienced team with specialized skills and the team we have at Peter Mac really are extraordinary at ensuring the best for the patients and trial compliance,” said Professor Desai.
KRAS is a key protein that controls how cancer cells divide and survive and when the KRAS-G12C protein mutates it makes cells, including cancer cells, more likely to divide uncontrollably leading to the development of tumors.
Peter Mac was also heavily involved in the design and conduct of a global phase I trial using divarasib on its own in advanced metastatic cancer published in the New England Journal of Medicine earlier this year.
More information:
Jayesh Desai et al, Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial, Nature Medicine (2023). DOI: 10.1038/s41591-023-02696-8
Journal information:
New England Journal of Medicine
,
Nature Medicine
Source: Read Full Article