Heterologous boosting of the whole-virion SARS-CoV-2 vaccine (CoronaVac, Sinovac) results in more robust immune responses than homologous boosting and could enhance protection, shows a recent study published in The Lancet and conducted by researchers from Brazil and the University of Oxford.
The most widely used vaccines in the ongoing coronavirus disease (COVID-19) pandemic were messenger RNA (mRNA), viral vector, and inactivated vaccines; however, widespread two-dose priming in low- and middle-income countries was primarily done with the use of whole-virion inactivated vaccines from Sinovac and Sinopharm.
Nonetheless, waning of immune responses has been documented after immunization with all available COVID-19 vaccines, which reduces protection against infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and results in the loss of protection against hospitalization and death (especially among older individuals).
After a regular schedule of receiving an inactivated vaccine, a third dose of CoronaVac (also known as a homologous boost) has been shown to be immunogenic. Still, there is a potential that boosting with a heterologous vaccine (which means another type of vaccine) might result in greater immunity and protection against SARS-CoV-2 variants of concern.
In the recently published study in The Lancet, first-authored by Dr. Sue Ann Costa Clemens from the University of Oxford in the United Kingdom and the University of Siena in Italy, a comprehensive analysis has been pursued on the immunogenicity and safety of both homologous and heterologous boosting after receiving the inactivated vaccine CoronaVac.
 Study: Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Image Credit: Viacheslav Lopatin / Shutterstock
Study: Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Image Credit: Viacheslav Lopatin / Shutterstock
A groupwise comparison
In this paper, volunteers (Brazilian adults older than 18) were divided into four groups to receive a booster dose of the Pfizer-BioNTech, Oxford-AstraZeneca Janssen, or CoronaVac coronavirus vaccines – six months after their previous immunizations with CoronaVac.
The primary study outcome was the non-inferiority of anti-spike immunoglobulin G (IgG) antibodies 28 days after the booster dose in the heterologous boost groups when compared to a homologous regimen. Secondary outcomes included neutralizing antibody titers at day 28, reactogenicity profiles, as well as adverse events.
It also has to be emphasized that antibody levels were low before the delivery of the booster dose, with only 20.4% of adults aged between 18 and 60 and 8.9% of adults aged over 60 presenting with detectable levels of neutralizing antibodies.
Increased neutralization titers
In a nutshell, there is robust evidence that the third dose of CoronaVac boosts the response and the production of neutralizing antibodies; however, these boosts were actually stronger with two different viral vector vaccines tested, while the highest antibody concentrations have been observed following an mRNA boost.
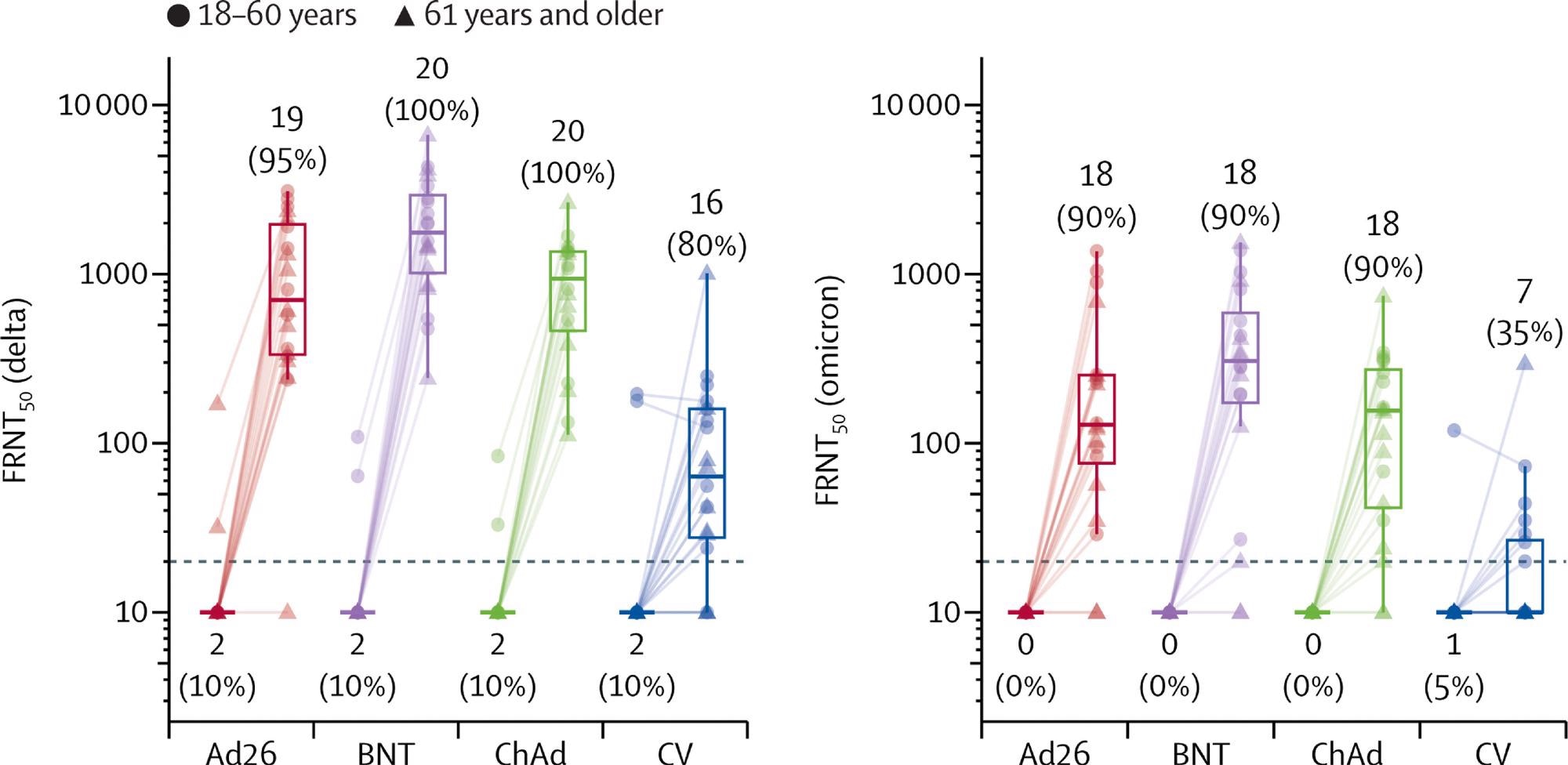 Live virus neutralization titers against delta and omicron variant strains, before and 28 days after boost vaccination by booster vaccine groups In each group, ten samples were selected from each age group (18–60 years, 61 years and older). Lines connect values from the same participant. The dotted line shows the lower limit of the assay. Values below the limit were substituted with a titer of 10. Participants with antibody titers above the lower limit are considered seropositive and are shown as percentages. The midlines of the boxes show medians and the outer bounds of the boxes show IQRs. Error bars extend to the last data point within 1·5 × the IQR above or below the 75th or 25th percentile. See appendix (p 14) for summary statistics. Ad26=Ad.26.COV2-S (n=20). BNT=BNT162b2 (n=20). ChAd=ChAdOx1 nCoV-19 (n=20). CV=CoronaVac (n=20). FRNT50=Focus reduction neutralization test—the reciprocal dilution of serum that neutralizes 50% of the input virus.
Live virus neutralization titers against delta and omicron variant strains, before and 28 days after boost vaccination by booster vaccine groups In each group, ten samples were selected from each age group (18–60 years, 61 years and older). Lines connect values from the same participant. The dotted line shows the lower limit of the assay. Values below the limit were substituted with a titer of 10. Participants with antibody titers above the lower limit are considered seropositive and are shown as percentages. The midlines of the boxes show medians and the outer bounds of the boxes show IQRs. Error bars extend to the last data point within 1·5 × the IQR above or below the 75th or 25th percentile. See appendix (p 14) for summary statistics. Ad26=Ad.26.COV2-S (n=20). BNT=BNT162b2 (n=20). ChAd=ChAdOx1 nCoV-19 (n=20). CV=CoronaVac (n=20). FRNT50=Focus reduction neutralization test—the reciprocal dilution of serum that neutralizes 50% of the input virus.
The researchers have also demonstrated that heterologous boosting increases live virus neutralization titers against both Delta (B.1.617.2) and Omicron (B.1.1.529) SARS-CoV-2 variants of concern. There were five serious adverse events among 1240 volunteers.
"The global priority remains first and second doses but this study provides important options for policymakers in the many countries where inactivated vaccines, like CoronaVac, have been used", emphasized Professor Sir Andrew Pollard, Director of the Oxford Vaccine Group and lead of the study in the press release from the University of Oxford.
Towards optimal booster programs
Findings in this study might be particularly relevant for the older adult population. However, it is still not fully clear how long immunity will persist after a third dose, so a follow-up period at six months in this study will enable a comparison of antibody waning across the four vaccines down the line.
"The new data presented here show the extraordinary response to the third dose of coronavirus vaccines in a heterologous vaccine schedule, supporting the recommendation of the Brazilian Ministry of Health to use RNA and viral vector vaccines for Brazil's booster program," said Professor Sue Ann Costa Clemens CBE of the Oxford Vaccine Group, and lead of the study in Brazil.
"These data will also guide other low- and middle-income countries in setting up the most optimal and affordable booster programs", she adds.
As new variants of concern may potentially emerge as the pandemic progresses, mixed vaccine schedules may be a way forward on how to achieve the strongest and optimal protection against not only the infection but also severe outcomes of COVID-19.
- Costa Clemens, S.A. et al. (2022). Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. The Lancet. https://doi.org/10.1016/S0140-6736(22)00094-0, https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)00094-0/fulltext
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Appendix, Assay, Coronavirus, Coronavirus Disease COVID-19, Homologous, immunity, Immunization, Immunoglobulin, Omicron, Pandemic, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Viral Vector, Virus

Written by
Dr. Tomislav Meštrović
Dr. Tomislav Meštrović is a medical doctor (MD) with a Ph.D. in biomedical and health sciences, specialist in the field of clinical microbiology, and an Assistant Professor at Croatia's youngest university – University North. In addition to his interest in clinical, research and lecturing activities, his immense passion for medical writing and scientific communication goes back to his student days. He enjoys contributing back to the community. In his spare time, Tomislav is a movie buff and an avid traveler.
Source: Read Full Article
