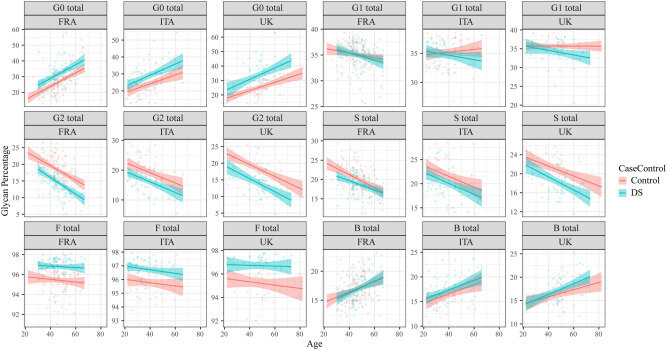
The molecular processes responsible for natural aging of cells are poorly understood. Studying conditions in humans where aging is accelerated due to genetic causes presents opportunities to learn about the mechanisms that control aging and devise strategies to slow down the aging process.
Adults who have Down syndrome (DS) show earlier signs of aging-related conditions: reduction in tissue regenerative capacity, alopecia, dry skin, delayed wound healing, chronic gum disease, osteoporosis, senescence of the brain and immune cells. DS is a genetic, but not inheritable condition, caused by being born with an extra copy of chromosome 21 (trisomy 21). It affects about 7 million people worldwide (around 60,000 in the U.K.).
DS is the most frequent genetic cause of intellectual disability and early onset Alzheimer’s disease. While increased risk of early Alzheimer’s is clearly caused by an extra copy of the amyloid precursor protein gene (APP) encoded on chromosome 21, the genetic basis for the other conditions is not easily explainable.
New research published in eBioMedicine, led by Queen Mary’s Professor Dean Nižetić and Dr. Aoife Murray, with collaborating institutions from Croatia, Singapore, France, Italy and four other London universities, has uncovered an overdosed gene on chromosome 21 causes cells of people with DS to age prematurely.
The study has shown that biological age of people who have DS is on average 19.1 years older than the chronologically age-matched people who don’t have DS. The research has also shown that this is not caused by co-morbidities of DS, and that the premature aging process starts very early in childhood. The gene for a kinase (a type of enzyme that speed chemical reactions in the body) called DYRK1A was identified as the main cause of the premature aging component of DS, showing that this gene’s overdose disturbs the DNA-damage-repair mechanisms, causing cells to develop more breaks in their DNA and fragility of their cell nuclei.
Dean Nižetić, professor of cell and molecular biology at Queen Mary, said, “We have uncovered that trisomic overdose of this gene (DYRK1A) is one of the main contributors to premature biological aging in DS. Further research is needed to understand how much this contributes to brain development and function, and also in finding ways of precisely inhibiting the overdose of this gene back to physiological levels. This could open exciting new possibilities for early interventions in DS, but a lot more research is needed.”
Carol Boys, chief executive of the Down Syndrome Association, said, “We have known for a long time that people who have Down syndrome experience an aging process which appears faster than in the general population.
To have a landmark research study like this, published by highly respected researchers, working together on an international basis is a pivotal development. Most importantly, the study hints at the prospect of effective treatments which may intervene in the accelerated cellular aging process. This aspect of the research will be of huge interest to people who have Down syndrome and their families.”
The research has also shown that genetic or chemical reduction of the action of this gene has the potential to correct the cellular aging defects. This opens possibilities for early therapeutic interventions for people with DS, to diminish the premature biological aging effects on their development and well-being. These findings also shed more light onto the natural mechanisms of aging and genes whose actions could be tackled to delay the natural aging process and reduce the risk of common aging-related diseases.
More information:
Aoife Murray et al, Dose imbalance of DYRK1A kinase causes systemic progeroid status in Down syndrome by increasing the un-repaired DNA damage and reducing LaminB1 levels, eBioMedicine (2023). DOI: 10.1016/j.ebiom.2023.104692
Journal information:
EBioMedicine
Source: Read Full Article