Malignant melanoma, a dangerous type of skin cancer, deploys an elegant molecular mechanism to evade natural immune responses and the therapies intended to boost them, according to new work led by scientists at the Herbert Irving Comprehensive Cancer Center (HICCC).
The findings, published in the current issue of Cancer Cell, explain how these tumors resist an important class of treatments, and suggests new strategies for attacking this and possibly other types of cancer as well.
Normally, the immune system eliminates abnormal cells, but cancers can elude it in various ways. For the past few years, Benjamin Izar, MD, Ph.D., assistant professor of medicine at Columbia University Vagelos College of Physicians and Surgeons and a member of the HICCC, has been studying how malignant melanoma, the deadliest form of skin cancer, hides itself from immune cells.
Initially, Izar’s team identified a signature pattern of gene expression changes associated with melanoma’s apparent cloaking device. “This signature was composed of a couple of hundred genes, so it was difficult to prioritize which of these may be the major functional driver,” says Izar.
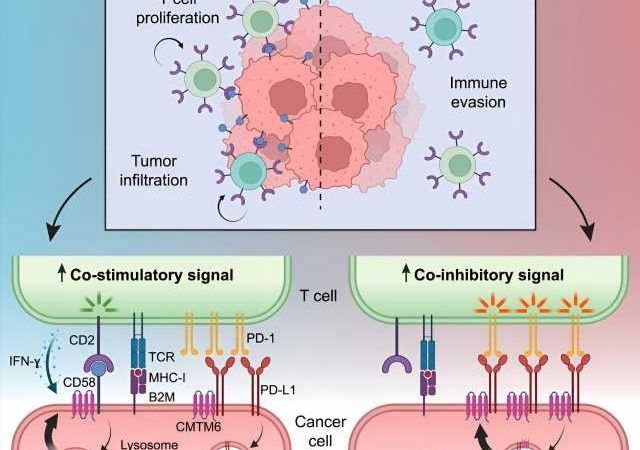
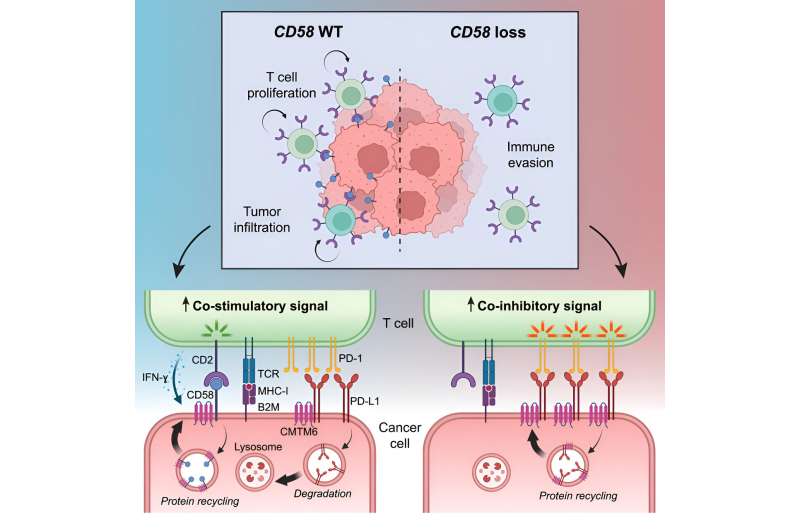
More recent experiments singled out a cell surface protein called CD58; in laboratory experiments, tumor cells that suppressed their CD58 expression got much better at escaping immune surveillance. CD58 stimulates T cells, white blood cells especially important for anti-tumor responses.
“The activity of T cells is regulated in a variety of ways, so they eliminate cancer or infected cells, but don’t go overboard causing damage to normal tissues,” says Izar. The findings hinted at how melanomas might be hiding from T cells, but didn’t provide the whole story. “This was the basis for asking ‘what are the mechanisms by which CD58 loss may drive cancer immune evasion?'” says Izar, who is the senior author on the new paper.
Izar’s team, including a network of collaborators at institutions across the U.S. and Europe, developed several new techniques to find the answer. To validate their earlier laboratory results, the scientists analyzed samples from melanoma patients before and during treatment with immune-targeting therapies, confirming that real tumors decrease CD58 expression while evading immunity.
Meanwhile, “Patricia Ho, an outstanding MD/Ph.D. student in my lab, performed a cool [molecular and cellular] analysis to identify, in an unbiased fashion, the key regulator of CD58: [the protein] CMTM6,” says Izar. The investigators also developed a special mouse model carrying specific human genes and cell types to see exactly how CD58, CMTM6, and other proteins operate in tumors facing the immune system in real time.
The results show that CMTM6 acts as a stabilizer, inhibiting the degradation of both CD58, which stimulates T cell activity, and another cell surface protein called PD-L1, which inhibits it. In fact, these two opposing signaling proteins compete with each other for CMTM6, so by reducing the amount of the pro-T cell CD58, tumor cells free up more of the CMTM6 to stabilize the anti-T cell PD-L1. “This is a double whammy to T cell activity: loss of activation and increased inhibition,” says Izar.
The work helps explain how malignant melanomas readily acquire resistance to immune-targeting therapies and points to ways drug developers might reverse the signaling pattern to restore the tumor’s vulnerability, exchanging its cloaking device for a high-visibility jumpsuit.
“We found, to our knowledge, the first example of a regulatory protein … that balances this yin and yang of immuno-stimulatory and inhibitory signals on cancer cells,” says Izar, adding that “it’s extremely likely that this mechanism is important in other cancers as well.”
More information:
Patricia Ho et al, The CD58-CD2 axis is co-regulated with PD-L1 via CMTM6 and shapes anti-tumor immunity, Cancer Cell (2023). DOI: 10.1016/j.ccell.2023.05.014
Journal information:
Cancer Cell
Source: Read Full Article