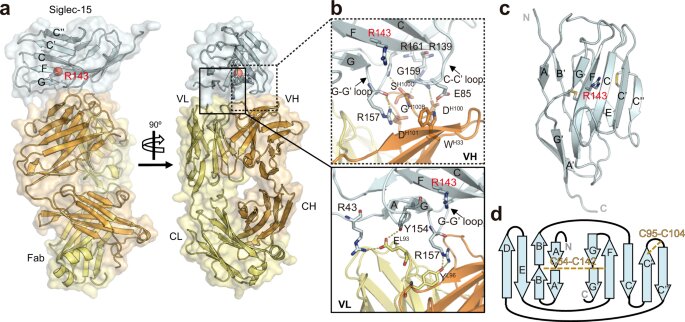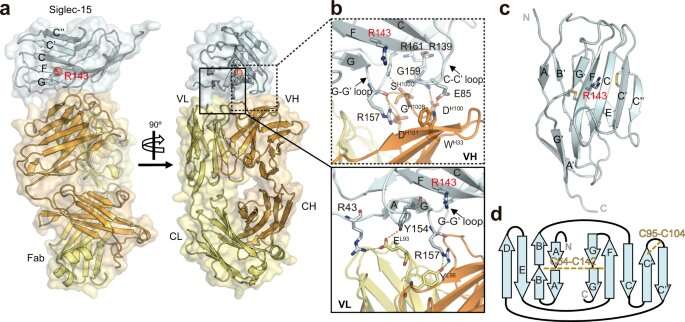
Siglec-15 has emerged as a promising target for therapeutic intervention in the field of cancer treatment, primarily due to its unique expression pattern and potential as an immune modulator. Its distinct expression profile, often found in tumor cells and tumor-associated macrophages, makes it an attractive candidate for targeted therapies aimed at modulating immune responses within the tumor microenvironment.
Despite the growing interest in Siglec-15, the development of effective drugs has been hampered by a limited understanding of its structural characteristics and the mechanisms by which it interacts with other molecules. In order to fully exploit the therapeutic potential of Siglec-15, a comprehensive understanding of its structure and molecular interactions is crucial.
A multidisciplinary research team from CIC bioGUNE, led by Jesús Jiménez Barbero, Asís Palazón and June Ereño, through a combination X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy and computational modeling elucidated the crystal structure of Siglec-15 and its binding epitope by co-crystallizing it with an anti-Siglec-15 blocking antibody. The research was first published online in the journal Nature Communications.
Using advanced techniques such as saturation transfer-difference nuclear magnetic resonance (STD-NMR) spectroscopy and molecular dynamics simulations, the team unravels Siglec-15’s binding mode to α(2,3)- and α(2,6)-linked sialic acids, as well as the cancer-associated sialyl-Tn (STn) glycoform.
“These results have revealed that the binding of Siglec-15 to T cells, which lack STn expression, depends on the presence of α(2,3)- and α(2,6)-linked sialoglycans. Additionally, the study identified the leukocyte integrin CD11b as a binding partner for Siglec-15 on human T cells. These discoveries provide a comprehensive understanding of Siglec-15’s structural features and underscore the crucial role of glycosylation in controlling T cell responses,” says June Ereño.
Prof. Jesús Jiménez Barbero emphasized the importance of glycosylation in understanding and manipulating immune responses and, together with all co-authors, they believe that this work has implications for the field of cancer immunotherapy as they offer valuable insights into the structure and interactions of Siglec-15. “This understanding opens up new possibilities for the design of next-generation immunotherapies targeting glycosylation or glycan-based pathways,” says Asis Palazón.
More information:
Maria Pia Lenza et al, Structural insights into Siglec-15 reveal glycosylation dependency for its interaction with T cells through integrin CD11b, Nature Communications (2023). DOI: 10.1038/s41467-023-39119-8
Journal information:
Nature Communications
Source: Read Full Article