Using laboratory-grown cells from humans and genetically engineered mice, scientists at Johns Hopkins Medicine say they have evidence that modifying a specific protein in immune white blood cells known as CD8+ T cells can make the cells more robust, potentially opening the door for better use of people’s own immune system T cells to fight cancer.
The findings were published in the journal JCI-Insight.
“Maximizing the effectiveness of T-cell-based therapies remains a critical challenge,” says David Kass, M.D., Abraham and Virginia Weiss Professor of Cardiology at the Johns Hopkins University School of Medicine and senior author of the study. “We’ve identified a powerful way to boost T cell function, offering a promising avenue for improving cancer immunotherapy and potentially treating a wide range of infectious and other diseases.”
Kass and his team emphasize that their experiments to date are preclinical, and will require significant further laboratory-based efforts before they can be applied to human therapies.
For the current study, Kass and his team focused on CD8+ T cells, the circulating immune system “soldiers” responsible for identifying and fighting infections and cancer cells.
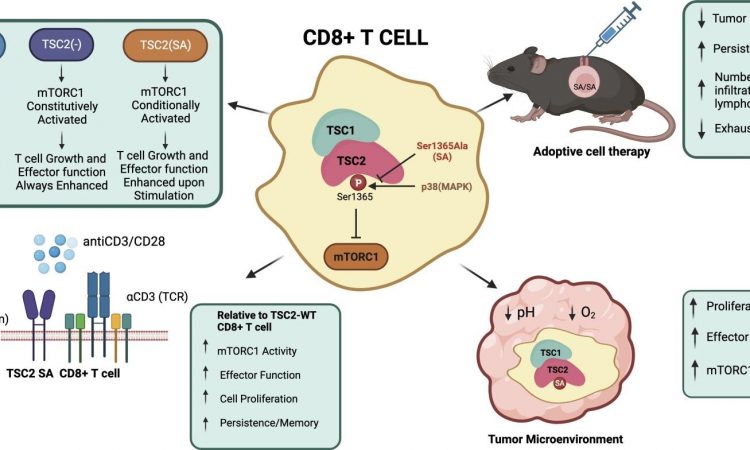
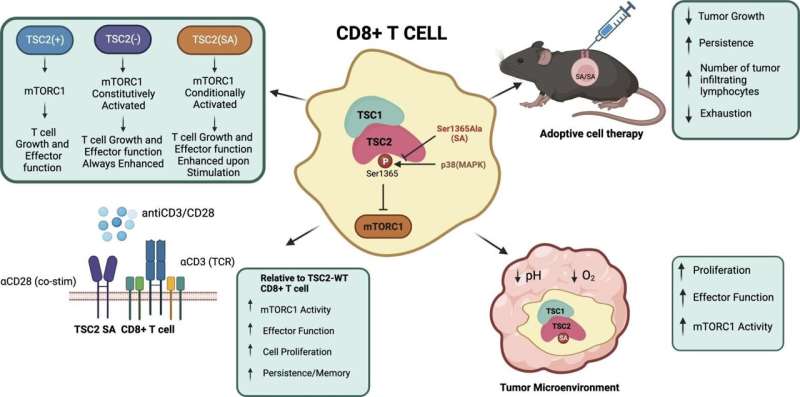
A protein called TSC2 (tuberous sclerosis protein 2) can activate or block a molecular pathway that regulates the T cells.
Overall, their experiments show that introducing a mutation in the TSC2 gene acts as a molecular “volume knob” to dial up or down the T cells’ regulatory pathway when it’s actively responding to immune challenges such as a virus or a cancer antigen. Like a volume knob, there is no change until the music starts—then it is louder.
The researchers found the mutated T cells were acting no differently than normal ones when they were just resting and not being stimulated to attack a target. They only became more active when stimulated. This type of control is relatively new for T cells.
This discovery, Kass says, raises the possibility of enhancing a therapy known as CAR-T, in which T cells are genetically engineered to better recognize a particular cancer. If these T cells also had the mutation in the TSC2 gene, the T cells could be more active against the tumor by multiplying, but could also persist longer, enhancing the T cells’ effectiveness to kill cancer.
The scientists also found that the T cells containing the TSC2 mutation could expand in great numbers during the initial immune response, but then could also persist long-term, which differs greatly from other T cells used for therapy.
The T cells with the TSC2 mutation were also better able to multiply and be activated to fight infections and to counter tumors, despite being in an environment that was more acidic or had lower oxygen than their nonmutated counterparts.
“After testing our modified T cells, we found they were better at stopping tumor growth, proliferated more inside the tumor and didn’t get tired out as easily,” Kass says. “As we continue to unravel the complexities of the immune system, such innovative approaches hold promise for revolutionizing the field of immunotherapy.”
In recent years, dozens of immunotherapies with novel drugs and antibodies have come on the market to treat cancer and other diseases, but response rates in patients vary widely, and overall, their optimal effectiveness as a group is only in the 15%–20% range. There is room for improvement, and many ongoing efforts are underway to do this. The new results may ultimately contribute to those efforts.
The researchers plan to do further studies with solid tumors such as lung, liver and colon cancers, which, compared with blood tumors such as leukemia, have been harder to achieve complete success with T cell-based therapies.
More information:
Chirag H. Patel et al, TSC2 S1365A mutation potently regulates CD8+T cell function and differentiation improving adoptive cellular cancer therapy, JCI-Insight (2023). DOI: 10.1172/jci.insight.167829 insight.jci.org/articles/view/167829
Journal information:
JCI Insight
Source: Read Full Article