A significant portion of mammalian genomes are composed of retrotransposable elements (REs). Unlike the previous hypothesis that these are “junk DNA,” recent studies have shown that REs such as endogenous retroviruses (ERVs) and long interspersed nuclear element 1 (LINE1) play essential roles in embryonic stem cells (ESCs) and during embryogenesis.
An increasing body of studies have shown that ERVs are aberrantly activated in a number of neurological disorders, and importantly, are directly involved in the pathological process of diseases.
The human Pogo Transposable Element Derived With ZNF Domain (POGZ) gene is one of the top risk genes mutated in neural developmental disorders, particularly autism spectrum disorder (ASD) and intellectual disability (ID). Unfortunately, the underlying pathology by POGZ dysfunction remains elusive. Thus, it is of great importance to understand the molecular function of POGZ.
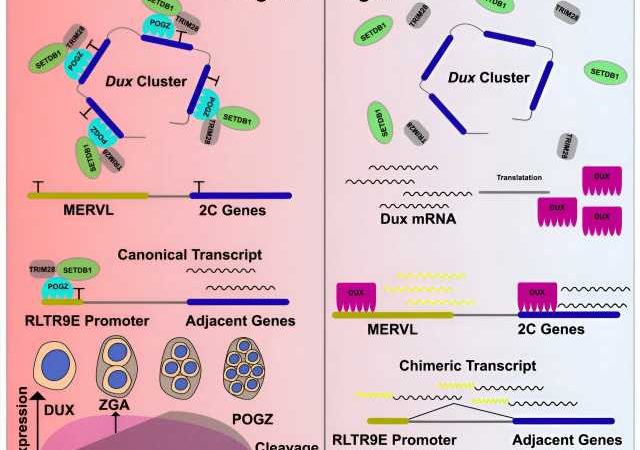
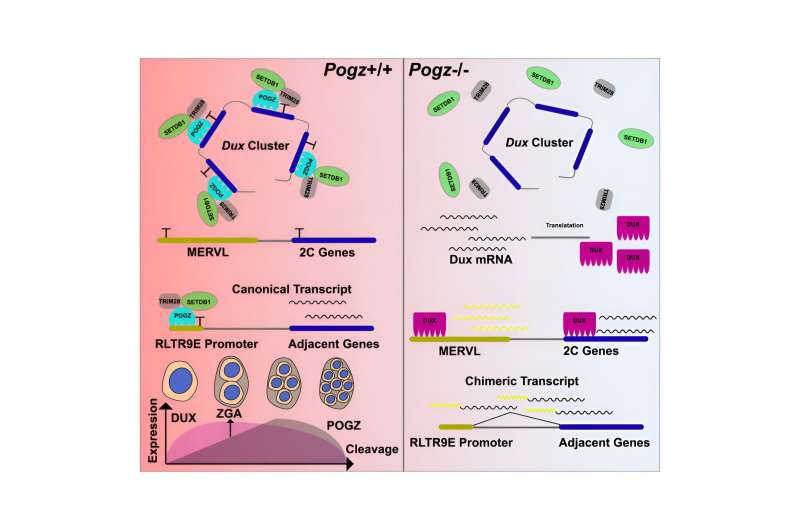
In a study published in Cell Reports, a research group led by Prof. Sun Yuhua from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences found that ASD-risk factor POGZ plays an important role in the maintenance of ESCs by silencing the Dux gene and ERVs.
The researchers generated POGZ mutant ESCs by using the CRISPR/Cas9 technology. POGZ depletion leads to up-regulation of 2C genes and the repetitive elements, resulting in transition to a 2C-like (2CLC) state and genome instability. By ChIP-seq and CUT&Tag, they demonstrated that POGZ directly occupied and repressed 2C master gene Dux and a subset of retrotransposon elements.
Additionally, the researchers found that POGZ was persistently required to repress ERVs during ESC neural induction, and chimeric transcripts were initiated within POGZ-bound ERVs and spliced to genic exons of neural genes. “These findings suggested that deregulation of nearby genes due to aberrant expression of POGZ-bound ERVs may contribute to the disease pathology in POGZ patients,” said Prof. Sun.
The study represents a significant advance in understanding POGZ function using the ESC model, and provides insights into understanding the neural disease mechanism caused by POGZ dysfunction.
More information:
Xiaoyun Sun et al, POGZ suppresses 2C transcriptional program and retrotransposable elements, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112867
Journal information:
Cell Reports
Source: Read Full Article