Northwestern Medicine investigators have discovered a novel mechanism that regulates neutrophil recruitment into tissue during inflammation, which could be targeted to treat different inflammatory diseases and conditions, according to a recent study published in the Journal of Clinical Investigation.
Neutrophils are specialized immune cells called effector cells that circulate in the bloodstream and upon an insult migrate from the blood vessels into surrounding tissues, where in greater numbers can exacerbate inflammation and tissue damage.
While neutrophil migration and signaling have been previously studied, the mechanisms that initiate this neutrophil recruitment response have remained unknown.
“That’s what we really tried to focus on is what cues will initiate this recruitment cascade,” said Ronen Sumagin, Ph.D., associate professor of Pathology in the Division of Experimental Pathology and senior author of the study.
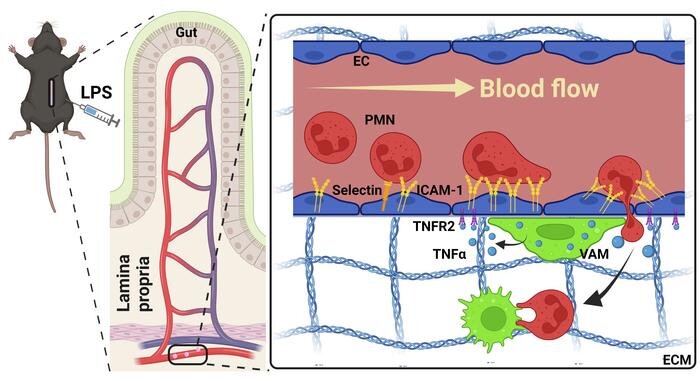
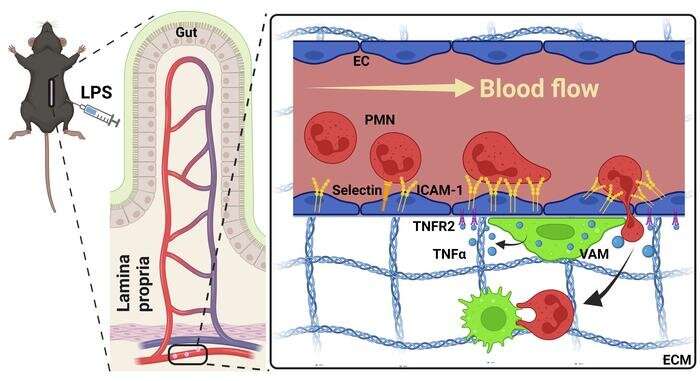
For neutrophils to migrate outside of the bloodstream and initiate an immune response, they must attach to endothelial cells that line the luminal side of blood vessel wall. In the current study, Sumagin’s team aimed to identify what exact mechanisms initiate these adhesive interactions.
“We’ve known for a long time that neutrophils do not cross the vessel wall everywhere; it’s not random. There are specific locations that are better equipped to support neutrophil crossing of the vessel wall, and what makes this so is actually really interesting. One neutrophil will go and then the second and third one will follow, and they really stick to these locations that we and others call ‘hotspots.’ That’s where they like to migrate, meaning these spots, for whatever reason, are better suited to accommodate recruitment than others,” Sumagin said.
Using real-time intravital microscopy to study blood vessels in inflamed intestinal tissue from mice, the investigators found that macrophages (specialized white blood cells) are recruited to endothelial cells which then activate specific mechanisms that tell neutrophils which locations, or “hotspots,” they can attach to on blood vessels.
During this recruitment process, macrophages will touch the blood vessel wall and then release an inflammatory cytokine called TNF-alpha, which attaches to a TNF receptor called TNFR2 on endothelial cells. This interaction then induces the expression of adhesion molecules on the surface of endothelial cells, such as ICAM-1, which in turn creates a hotspot.
“This combination of a macrophage touch and endothelial cell activation and an induction of ICAM creates a hotspot for neutrophils to then migrate… so we think that macrophages drive this hotspot formation and, in a way, serve as gate keepers of neutrophil migration,” Sumagin said.
These newly identified mechanisms could be a potential target for treating different inflammation-driven diseases through regulating the neutrophil recruitment process, according to Sumagin.
“We can now try to target macrophage-endothelial cell communication to either prevent or promote the formation of those hotspots,” Sumagin said.
More information:
Xingsheng Ren et al, Macrophage-endothelial cell crosstalk orchestrates neutrophil recruitment in inflamed mucosa, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI170733
Journal information:
Journal of Clinical Investigation
Source: Read Full Article