Researchers revealed the importance of the transmission of epigenetic information through cell division for embryonic stem cells functionality. This has broad implications for aging, cancer, and regenerative medicine.
A groundbreaking study from the University of Copenhagen has shed light on the significance of transmitting epigenetic information during cell division for proper function of the so-called embryonic stem cells, cells in a dish that resemble cells from very early embryo which can recapitulate development.
“Establishing a mechanistic understanding for how cells and their descendants retain their identity and why this memory is important for their capacity to change,” says Anja Groth, Professor at Novo Nordisk Foundation Center for Protein Research.
“These processes are at the root of how cells can maintain optimum function as the organism ages, and therefore, understanding the processes we describe here could lead to new approaches to combat functional decline associated with aging and cancer.”
The many trillions of cells in our body have the same genetic information, but epigenetic information dictates what specific genes each type of cell (nerves, muscles, skin, etc.) expresses and has the potential to communicate this information to its daughter cells.
In this study the researchers seek to explain epigenetic inheritance transmitted by histones and how they support the epigenome and cell identity.
“Understanding the layers which control epigenetic memory could be a key to aging and cancer avoidance as well as facilitating more efficient cellular reprogramming and trans-differentiation for applications regenerative medicine,” says Joshua Brickman, Professor at Novo Nordisk Foundation Center for Stem Cell Medicine, reNEW Copenhagen.
The study has been done on embryonic stem cells from mice, but according to the researchers it gives a good understanding of how the mechanism work in human cells as well because these mechanisms are fundamental for all living cells.
The role of genetic information
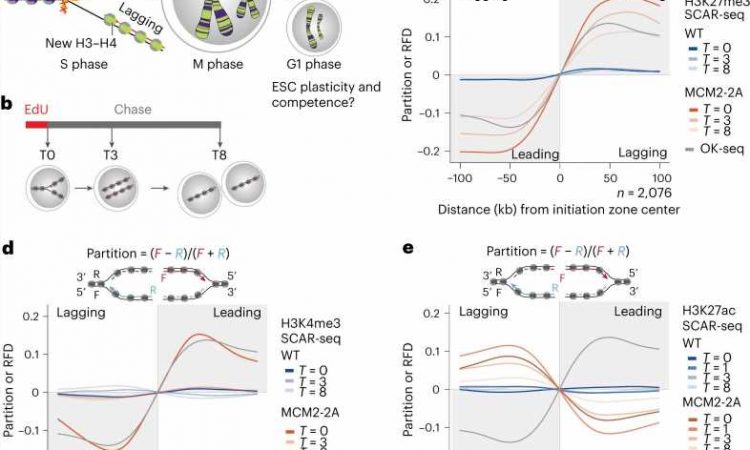
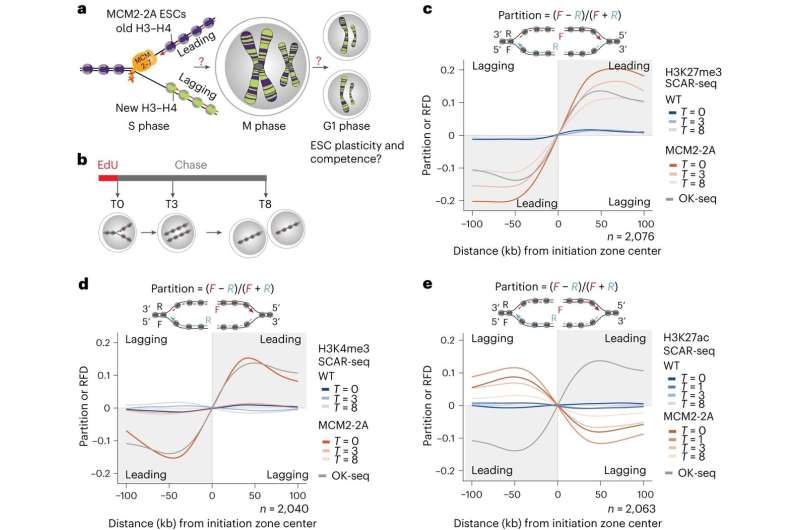
The Groth laboratory (CPR) leading this study also recently discovered that histones are transmitted during DNA replication by the same machinery that copies the genetic code.
“By using this information, we were able to manipulate the machinery to create mouse embryotic stem cells, where one daughter DNA strand lacks the transmitted histones with the memory signal while the other gets it all,” says postdoc at Novo Nordisk Foundation for Protein Research Alva Biran and co first author of the study.
That put the researchers in a unique position, allowing them to answer the long-standing question regarding the role of transmitted non-genetically coded information. That is important because the mother cell transmits a precise amount of chemically modified histones to its daughter cells, which the investigator found to enable the balanced inheritance of functional properties associated with cell identity.
“Once the transmission of information is disrupted, the epigenome losses its fidelity resulting in alterations to gene expression that are dependent on the transmission of the memory signals on the H3-H4 histones, and it results in cells losing their competence for embryonic development,” says Anja Groth.
Better possibilities for regenerative medicine
In this study the researchers focus on stem cells, but the research itself represents the first demonstration that this sort of inheritance is essential for cell function and differentiation.
“This study holds significant implications for both physiological processes such as organismal development and stable maintenance of adult tissues at the same time as providing important clue to what could go wrong in disease,” says Joshua Brickman.
“Moreover, we hope to be able to manipulate these processes as a means to develop better cell-based products and treatments for regenerative medicine.”
The paper is published in Nature Genetics.
More information:
Alice Wenger et al, Symmetric inheritance of parental histones governs epigenome maintenance and embryonic stem cell identity, Nature Genetics (2023). DOI: 10.1038/s41588-023-01476-x
Journal information:
Nature Genetics
Source: Read Full Article