A recent research paper by scientists from Denmark reveals that circulating plasmacytoid dendritic cells may be a potential therapeutic target to maintain desired levels of an antiviral compound known as interferon, allowing for the mitigation of coronavirus disease (COVID-19) severity. The study is currently available on the bioRxiv* preprint server while it undergoes peer review.
Akin to other viruses, highly pathogenic coronaviruses have a sundry of different strategies to interfere with the host’s immune response and pursue immune evasion, which is linked to viral pathogenicity. Hence, a complete understanding of how the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) avoids immune responses is pivotal for the development of antiviral treatments.
Since the start of the COVID-19 pandemic, many studies have hinted that different cell types (but also diverging sensing pathways) may be responsible for controlling viral infection, but also for the surge in inflammatory cytokines that are characteristic for the infected patients.
In our immune system, plasmacytoid dendritic cells are autonomous producers of type I interferon-alpha, making them one of the key players in controlling viral infections. Clinical studies have shown that severe COVID-19 cases have a reduction in circulating plasmacytoid dendritic cells, as well as minimal influx of these cells into the lungs (when compared to patients with moderate forms of the disease and healthy controls).
However, it is unclear whether disease severity arises due to the lack of plasmacytoid dendritic cells in the lungs or as a result of dysfunctional cytokine production. In addition, the mechanism of how these cells may sense SARS-CoV-2 has not been determined.
Screening via CRISPR-editing approach
The study, first-authored by Renée M. van der Sluis from the Aarhus University in Denmark, aimed to explore the exact molecular mechanism that plasmacytoid dendritic cells utilize in order to sense SARS-CoV-2 once the virus enters a human body.
The research group used the CRISPR gene editing approach to screen for several innate immune sensor pathways that are implicated in the production of antiviral interferons and inflammatory cytokines upon viral sensing. For that purpose, blood samples from patients hospitalized due to COVID-19 were collected at hospital admission.
The investigation of sensing mechanisms has been done using a cellular platform designed to generate human primary plasmacytoid dendritic cells ex vivo with the help of hematopoietic stem and progenitor cells from healthy individuals.
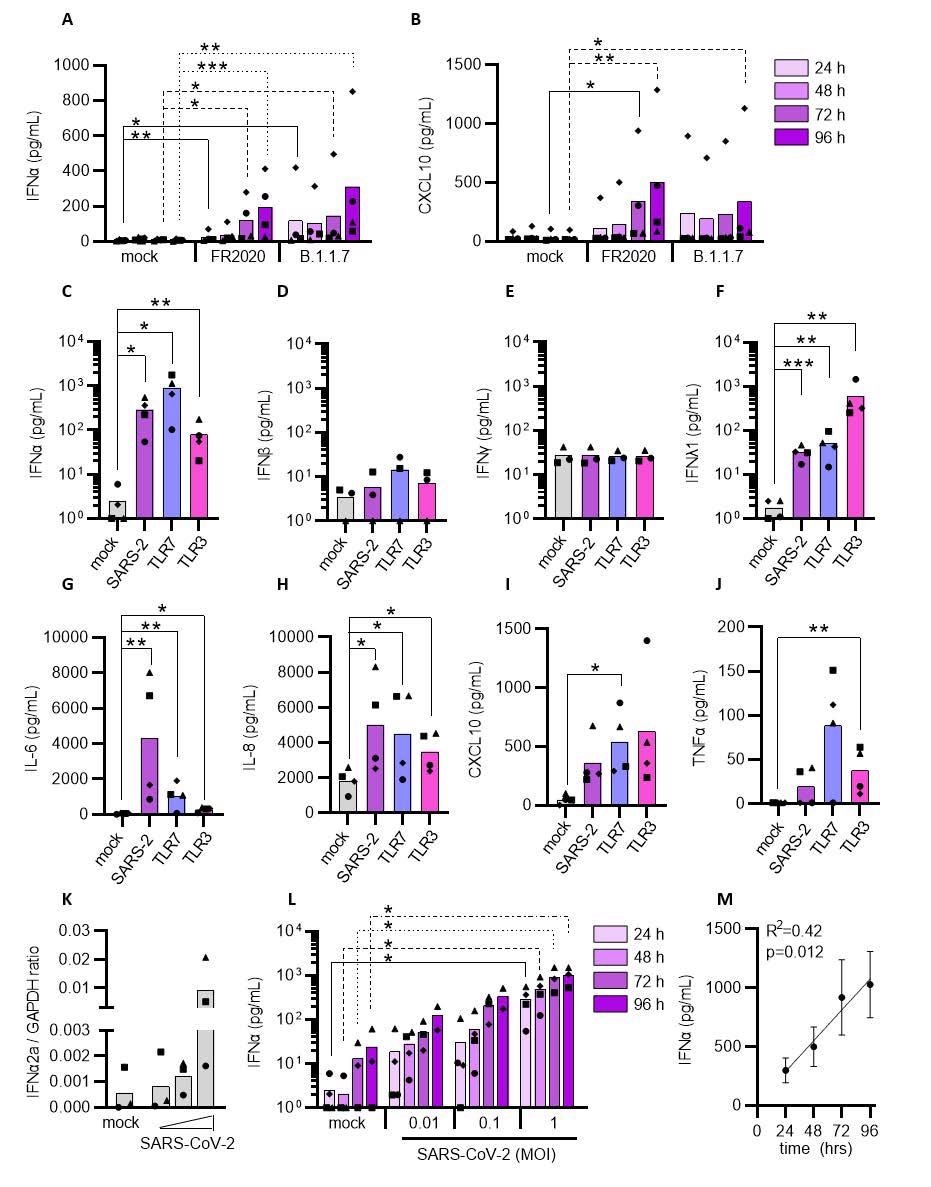
Furthermore, a broad exploration of the timing and nature of SARS-CoV-2-induced antiviral responses in plasmacytoid dendritic cells was done by profiling 789 selected genes (covering major immunological pathways) with the use of the NanoString nCounter technology (i.e., a system for digitally detecting and enumerating large sets of molecules).
Sensing SARS-CoV-2 and producing cytokines
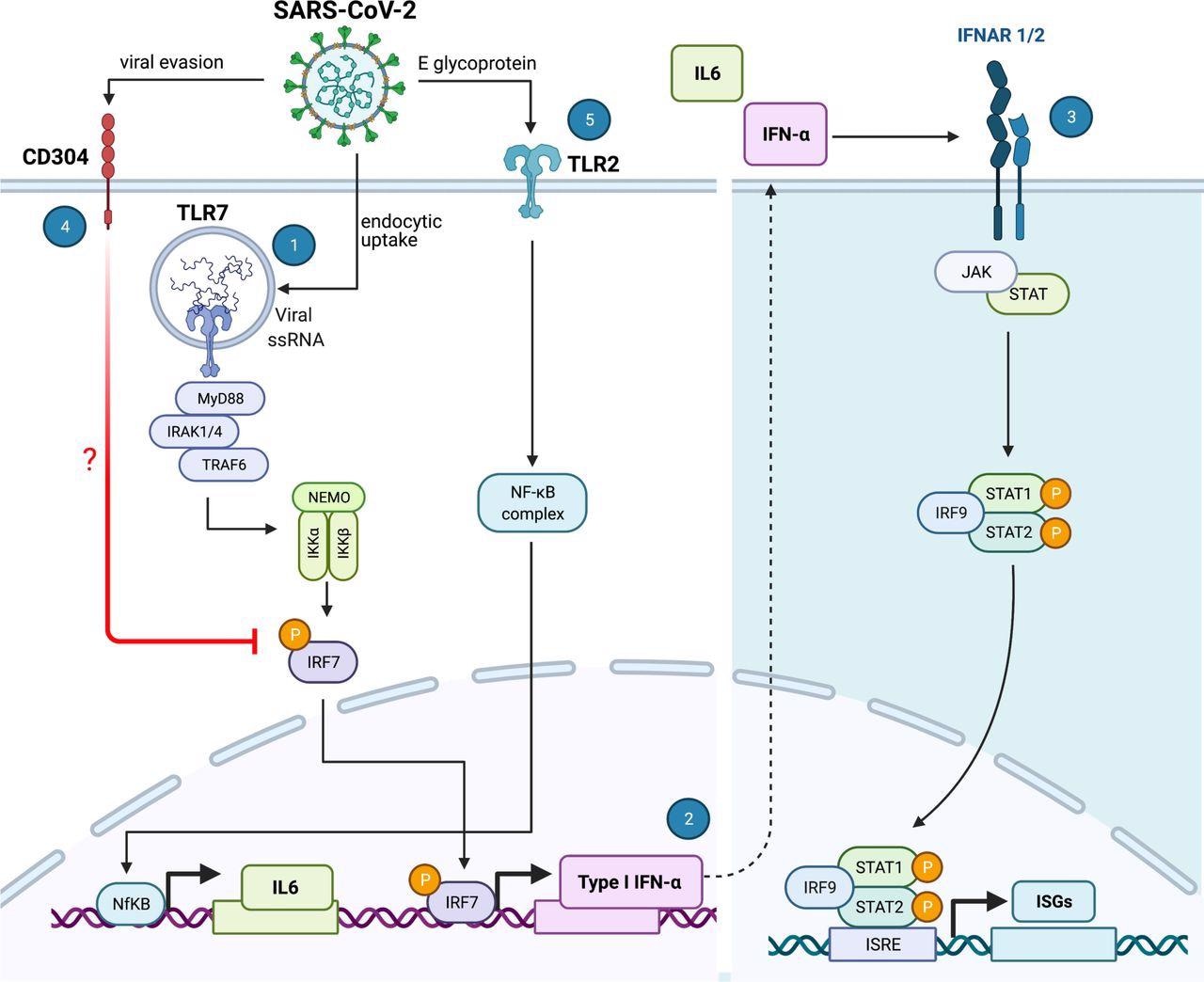
In short, the researchers have shown that plasmacytoid dendritic cells are capable of sensing SARS-CoV-2 and, in response, produce type I interferon-alpha, prompting, in turn, the production of inflammatory cytokines that give rise to the cytokine storm observed in people suffering from severe forms of COVID-19.
More specifically, plasmacytoid dendritic cells sense SARS-CoV-2 and elicit antiviral protection of lung epithelial cells through Toll-like receptor 7 (TLR7), while recognition of Toll-like receptor 2 (TLR2) elicits an IL-6 inflammatory response associated with immune pathology.
Furthermore, this study emphasizes that SARS-CoV-2 utilizes neuropilin-1 not only as an alternative receptor to the angiotensin-converting enzyme 2 (ACE2) for viral entry but also to mitigate the production of type I interferon-alpha by plasmacytoid dendritic cells – reducing the host’s innate antiviral immune response.
A potential treatment target
The results of this study highlight distinct sensing pathways used by plasmacytoid dendritic cells to prompt antiviral vs. immunopathological responses to SARS-CoV-2 and suggest that targeting neuropilin-1 may be clinically relevant for mounting TLR7-mediated antiviral protection.
“Here we show that in COVID-19 patients, circulating plasmacytoid dendritic cells decline early after symptom onset and this correlated with COVID-19 disease severity”, emphasize study authors in this bioRxiv paper.
In conclusion, this study provides evidence that these circulating could be a potentially promising treatment target that will preserve desired antiviral interferon levels and allow for the mitigation of COVID-19 severity.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- van der Sluis, R.M. et al. (2021). Distinct SARS-CoV-2 sensing pathways in pDCs driving TLR7-antiviral vs. TLR2-immunopathological responses in COVID-19. bioRxiv. https://doi.org/10.1101/2021.11.23.469755, https://www.biorxiv.org/content/10.1101/2021.11.23.469755v1
Posted in: Medical Research News | Medical Condition News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Blood, Cell, Compound, Coronavirus, Coronavirus Disease COVID-19, CRISPR, CXCL10, Cytokine, Cytokines, Enzyme, Ex Vivo, Gene, Genes, Glycoprotein, Hospital, Immune Response, Immune System, Interferon, Interferons, Intracellular, Lungs, Pandemic, Pathology, Phosphorylation, Progenitor Cells, Protein, Receptor, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, TNFα, Virus

Written by
Dr. Tomislav Meštrović
Dr. Tomislav Meštrović is a medical doctor (MD) with a Ph.D. in biomedical and health sciences, specialist in the field of clinical microbiology, and an Assistant Professor at Croatia's youngest university – University North. In addition to his interest in clinical, research and lecturing activities, his immense passion for medical writing and scientific communication goes back to his student days. He enjoys contributing back to the community. In his spare time, Tomislav is a movie buff and an avid traveler.
Source: Read Full Article
