Comorbidities and various risk factors like age, obesity, chronic respiratory disease, and cardiovascular disease affect the severity of coronavirus disease 2019 (COVID-19). In addition, neurological symptoms are one of the common symptoms of COVID-19, which indicates that the virus can potentially infect and replicate in the central nervous system (CNS). However, various pieces of evidence prove that the virus does not exhibit wide neuroinvasive properties.
Encephalitis and meningitis have been reported in several COVID-19 patients, and viral RNA and protein have also been identified within the CSF of infected patients. Although human brain organoids are vulnerable to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, extensive CNS penetrance by SARS-CoV-2 has not been demonstrated. Pre-clinical animal models such as human angiotensin-converting enzyme 2 (hACE2) transgenic mouse models have provided key insights into COVID-19 pathogenesis.
Intranasal SARS-CoV-2 infection of K18-hACE2 transgenic mice to study the neurological impact of SARS-CoV-2
In a study recently published on the bioRxiv* preprint server, researchers attempted to further increase the scope of previous studies in examining SARS-CoV-2 infection of human CNS resident cells. They evaluated the immune response to SARS-CoV-2 infection of the CNS of K18-hACE2 mice and assessed the influence of microglia in host defense following CNS infection by SARS-CoV-2.
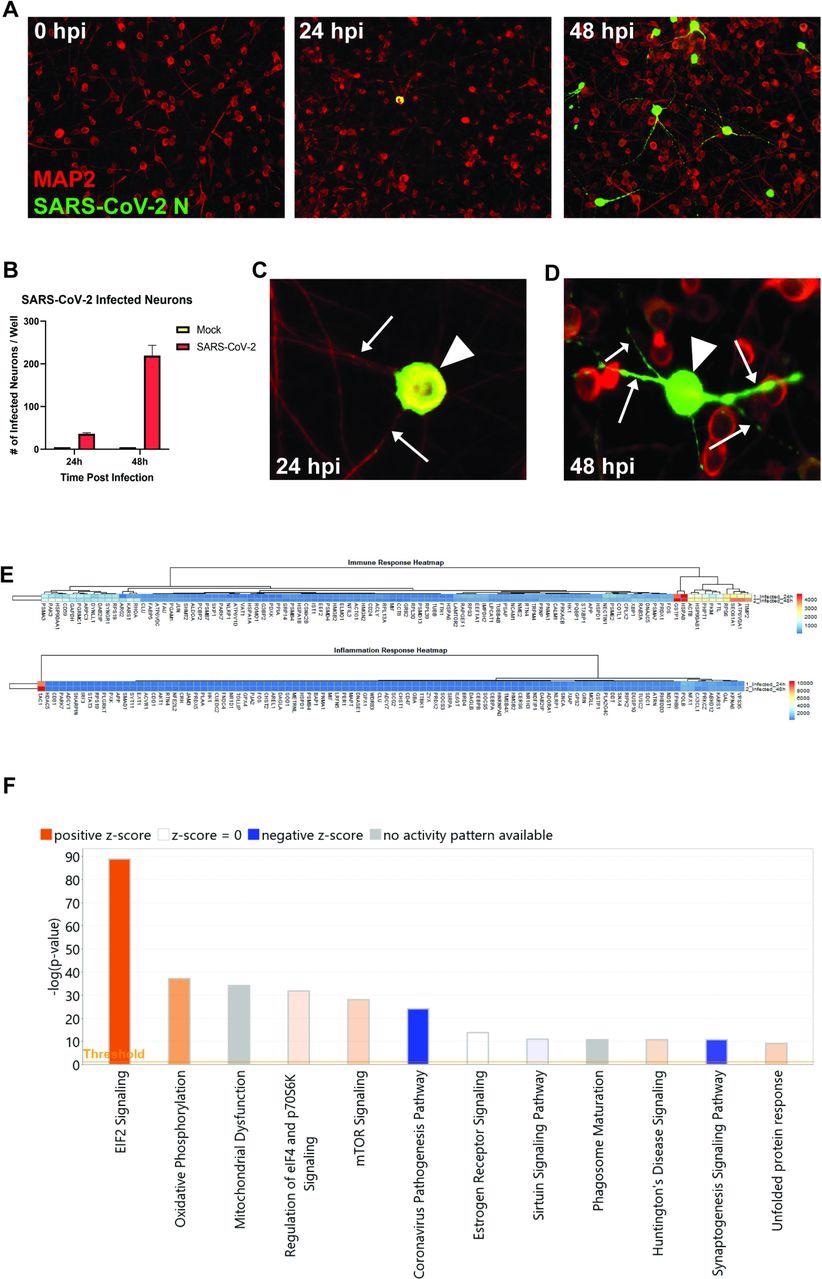
The human iPSC-derived neurons were inoculated with SARS-CoV-2. The staining of nucleocapsid proteins confirmed that SARS-CoV-2 was able to infect and replicate within neurons. The observations suggested that the virus may not spread via fusion with neighboring cells since syncytia formation was not detected in neuron cultures. Although the Coronavirus Pathogenesis Pathway was overrepresented, it was inhibited in response to neuronal infection by SARS-CoV-2.
SARS-CoV-2 infection of K18-hACE2 mice
K18-hACE2 mice were intranasally infected with plaque-forming units (PFU) of SARS-CoV-2, and the resulting weight loss and mortality were recorded.
The findings showed the development of interstitial pneumonia and immune cell infiltration associated with viral RNA present within the lungs.
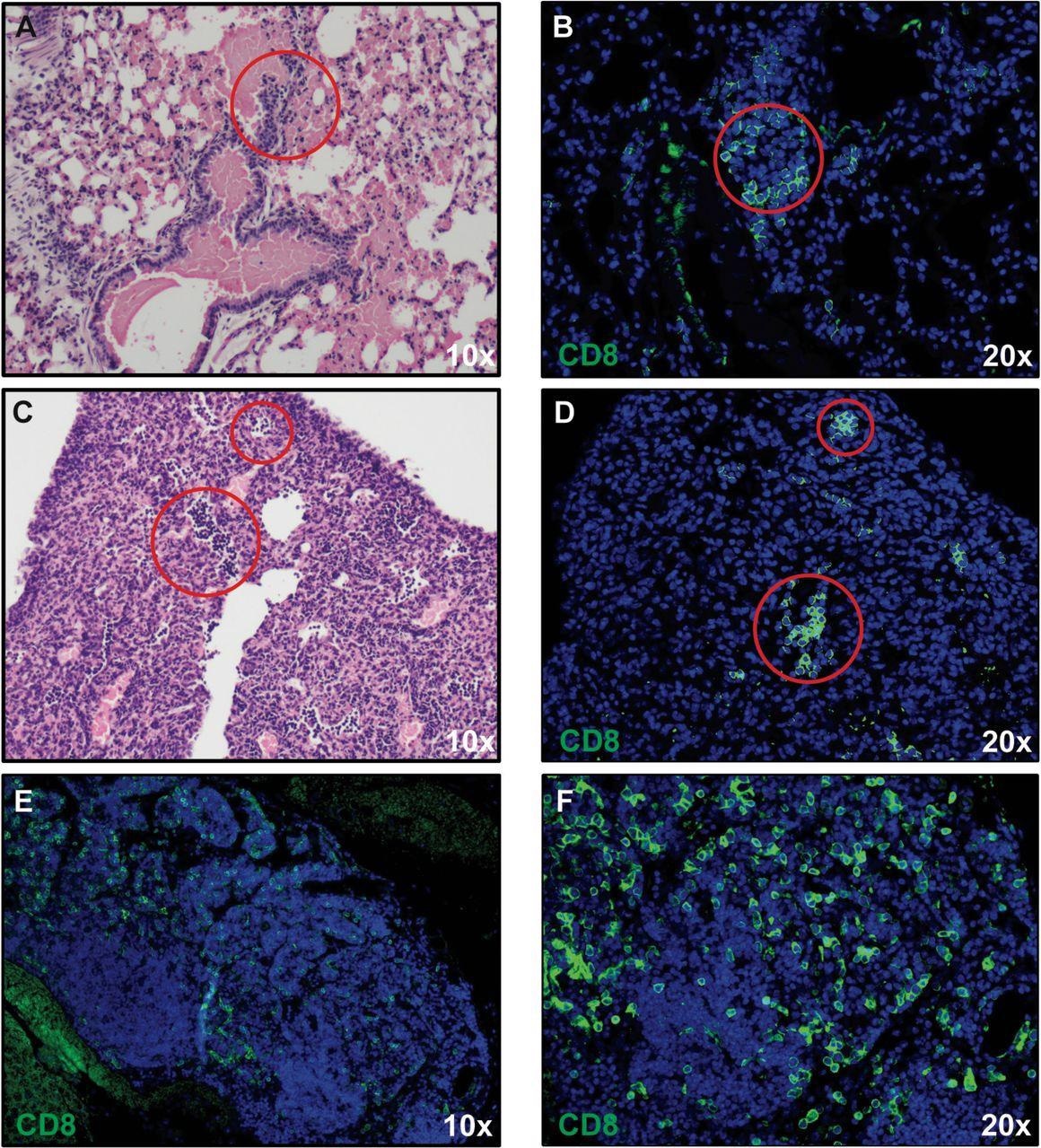
The expression of proinflammatory cytokines (Ifn-λ and Tnf-α)/chemokines (Cxcl9, Cxcl10, Ccl2, Ccl5, and Ccl19) in response to infection increased, which correlated with microgliosis and the presence of inflammatory cells.
The researchers identified inflammatory CD8+ T cells in the lungs of infected mice, which were presumably responding to the T cell chemoattractant CXCL10.
In the same way, inflammatory monocyte/macrophages were likely employed in response to the expression of CCL2. The presence of inflammatory neutrophils was likely reflected due to the increased expression of transcripts encoding CXCR2.
It was observed that, in SARS-CoV-2-infected mice, microglia depletion via administration of colony-stimulating factor 1 receptor inhibitor PLX5622 did not affect survival or viral replication. However, the expression of proinflammatory cytokine/chemokine transcripts was dampened, and a reduction in monocyte/macrophage infiltration was observed.
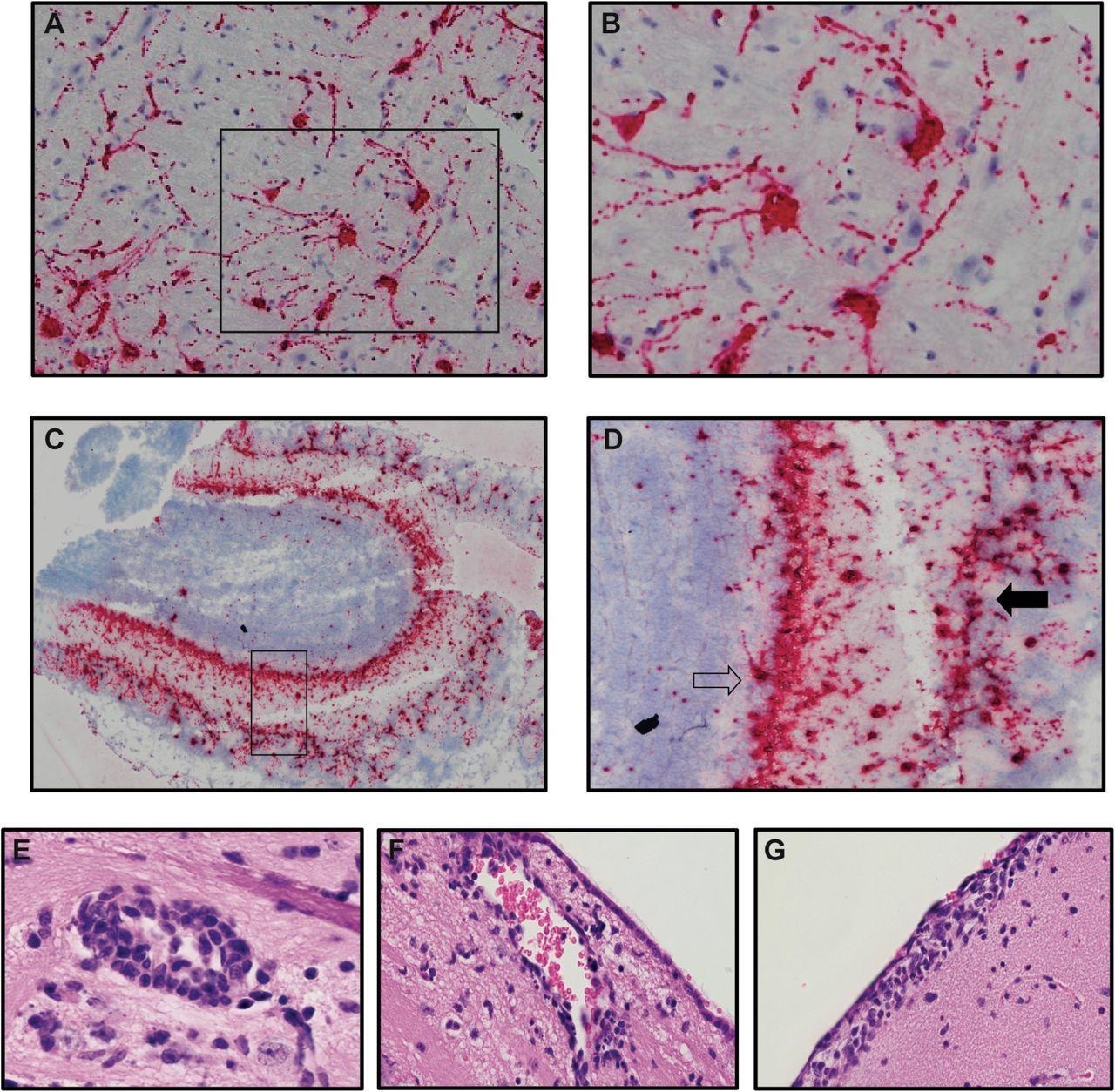
The outcomes of the study claim that microglia did not have a role in SARS-CoV-2 replication in the K18-hACE2 model but contributed to an inflammatory response through the expression of proinflammatory genes.
Conclusions
Microglia plays an important role in host defense in response to viral infection of the CNS. Ablation of microglia through CSF1R inhibition causes increased mortality in mice infected with West Nile Virus (WNV) and was correlated with weakened activation of antigen-presenting cells (APCs) and limited reactivation of virus-specific T cells that leads to reduced viral clearance.
This study showed that microglia depletion in SARS-CoV-2 infected mice did not have an impact on viral survival or replication but did lead to dampened expression of proinflammatory cytokine/chemokines and reduced monocyte/macrophage infiltration.
The study's findings add to previous reports indicating the ability of SARS-CoV-2 to infect neurons and highlight the potential use of the K18-hACE2 model to study immunological and neuropathological aspects related to SARS-CoV-2-induced neurologic disease.
“These findings emphasize the importance of working with animal models in which SARS-CoV-2 entry into the CNS is more consistent with what has been observed in COVID-19 patients.”
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Microglia do not restrict SARS-CoV-2 replication following infection of the central nervous system of K18-hACE2 transgenic mice. Gema M. Olivarria, Yuting Cheng, Susana Furman, Collin Pachow, Lindsay A. Hohsfield, Charlene Smith-Geater, Ricardo Miramontes, Jie Wu, Mara S. Burns, Kate I. Tsourmas, Jennifer Stocksdale, Cynthia Manlapaz, William H. Yong, John Teijaro, Robert Edwards, Kim N. Green, Leslie M. Thompson, Thomas E. Lane, bioRxiv, 2021. DOI: https://doi.org/10.1101/2021.11.15.468761, https://www.biorxiv.org/content/10.1101/2021.11.15.468761v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Angiotensin, Angiotensin-Converting Enzyme 2, Antigen, Brain, Cardiovascular Disease, CCL19, CCL2, CCL5, Cell, Central Nervous System, Chemokine, Chemokines, Chronic, Coronavirus, Coronavirus Disease COVID-19, Cortex, CXCL10, Cytokine, Cytokines, Encephalitis, Enzyme, Fluorescence, Genes, heat, Hybridization, Immune Response, Inflammation, Lungs, Lymph Node, Macrophage, Magnification, Meningitis, Microglia, Monocyte, Morphology, Mortality, Nervous System, Neuron, Neurons, Neutrophils, Obesity, Organoids, Pneumonia, Protein, Receptor, Respiratory, Respiratory Disease, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Transgenic, Virus, Weight Loss, West Nile Virus

Written by
Saurabh Chaturvedi
Saurabh Chaturvedi is a freelance writer from Jaipur, India. He is a gold medalist in Masters in Pharmaceutical Chemistry and has extensive experience in medical writing. He is passionate about reading and enjoys watching sci-fi movies.
Source: Read Full Article
