In a recent study under consideration at Scientific Reports and posted to the Research Square* preprint server, researchers evaluated the impact of time on the sensitivity of four severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serological assays using samples from the coronavirus disease 2019 (COVID-19) staff testing of antibody responses study (Co-Stars) in the United Kingdom (UK).
Following the emergence of SARS-CoV-2 in late 2019, a wide range of serological tests has been developed to estimate the seroprevalence of SARS-CoV-2, eligibility for COVID-19 booster vaccinations, and anti-SARS-CoV-2 antibody therapies. The regulatory authorities approved the SARS-CoV-2 serological assays after evaluating their performances two to three weeks after infection on reference sera samples, including SARS-CoV-2-negative or positive controls.
Further, the SARS-CoV-2 serological analyses are essential to understand the immune correlates of protection from future COVID-19 waves following natural infection or vaccination. Nonetheless, there is only limited knowledge about the performance of these serological tests over time following SARS-CoV-2 infection.

Study: The Influence of Time on the Sensitivity of SARS-CoV-2 Serological Testing. Image Credit: Andrei Dubadzel / Shutterstock.com
About the study
In the study, the researchers concurrently compared the results of the multiplexed spike (S), nucleoprotein (N) and receptor-binding domain (RBD) Meso Scale Discovery (MSD) assay, the Roche-S assay, the Roche-N assay, and the Abbott-N assay on monthly samples from healthcare workers (HCWs) for 250 days following SARS-CoV-2 infection procured in connection with Co-Stars.
Initially, the sera samples from 3657 subjects were screened using enzyme-linked immunosorbent assay (ELISA), and then samples were collected monthly from those HCWs who were SARS-CoV-2-positive up to approximately six months.
The team evaluated the proportion of samples that remained SARS-CoV-2 seropositive as time passed by survival analysis. They further estimated the decay rate of the SARS-CoV-2 S antibody and the N antibody for the four diagnostic assays employing a previously published mathematical model fitted to the current data.
Study findings
The results show that nearly 98% of the subjects were SARS-CoV-2-seropositive by two or more assays evaluated. The quantitative Roche-S and MSD assays indicated that the SARS-CoV-2 S antibody production remained high for about six months after the infection. However, all N antibody assays showed the deterioration of the SARS-CoV-2 N antibodies with time. The waning effect of the N antibody was slower in the Roche-N assay than in the Abbott-N assays.
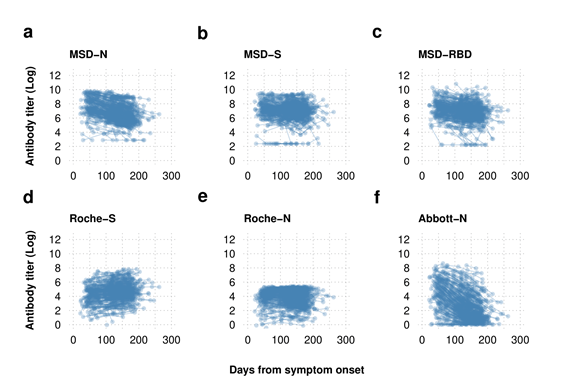
Log transformed serial serological antibody titer data plotted by time from symptom onset Antibody dynamics are dependent on the assay used with the sensitive Roche-S and MSD-S assay demonstrating maintenance of the spike protein antibody while the nucleoprotein antibody is shown to wane with the MSD and Abbott-N assays but to a lesser extent with the Roche-N assay.
Every assay evaluated in the current study demonstrated significant sensitivity following 50 days of SARS-CoV-2 infection. While almost half of the Abbott-N assays exhibited a SARS-CoV-2-negative result following 175 days of COVID-19, the results of the semi-quantitative Roche-N assay and the MSD-N assay remained seropositive. Further, the Roche-S and the MSD-S assays also maintained the SARS-CoV-2 seropositivity up to 200 days following the infection.
The MSD-RBD assay inferred some evidence for reductions of SARS-CoV-2-seropositivity with time. However, the top-performing quantitative Roche-S assay indicated no proof of the waning of S antibody titers and even a slight increase after 200 days of symptom onset of the SARS-CoV-2 infection.
Of 183 SARS-CoV-2-seropositive samples from the quantitative MSD-N assay with an antibody titer value less than 403, 137 were seronegative by the Abbott-N assays. Hence, suggesting the Abbott-N assays were not suitable for seroprevalence investigations. The deterioration in the performance of the Abbott-N assays was associated with the decline of detectable antibody titers over time.
Conclusions
According to the researchers, this is the first study evaluating the sensitivity of various COVID-19 diagnostic tests using longitudinally procured serological samples from SARS-CoV-2-infected patients in parallel. The study findings indicate that although several SARS-CoV-2 serological analyses demonstrated high sensitivity during the initial three weeks following the infection, it was not the case six months after COVID-19.
In detail, the study showed that the Abbott-N assay failed to detect the SARS-CoV-2 antibodies over time following the infection. By contrast, the MSD and the Roche assays demonstrated high sensitivity up to 200 days following SARS-CoV-2 infection. This suggests that the sensitivity of SARS-CoV-2 serological testing varies depending on the assay technique used.
Collectively, the study highlights the significance of caution while using the Abbott assay in SARS-CoV-2 seroprevalence investigations and clinical decision-making, such as determining the eligibility for anti-SARS-CoV-2 antibody therapy or COVID-19 booster vaccination due to its reducing SARS-CoV-2 sensitivity as time elapses.
Moreover, the presence of the neutralizing S antibodies is linked to lower chances of SARS-CoV-2 re-infection and severe form of the disease. Therefore, the long-lasting detectable SARS-CoV-2 neutralizing S antibody titers by the quantitative Roche-S assay adds on the proof for the prolonged protection against the pre-existing strains of SARS-CoV-2 after the natural infection.
Furthermore, the study indicates the applicability of the Roche-S assay in diagnosing multisystem inflammatory syndrome in children (MIS-C), long coronavirus disease (COVID), and SARS-CoV-2 following vaccination.
*Important notice
Preprints with Research Square publish preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Arturo Torres Ortiz, Fernanda Fenn Torrente, Adam Twigg et al. The Influence of Time on the Sensitivity of SARS-CoV-2 Serological Testing, 17 February 2022, PREPRINT (Version 1) available at Research Square https://doi.org/10.21203/rs.3.rs-1286644/v1, https://www.researchsquare.com/article/rs-1286644/v1
Posted in: Device / Technology News | Medical Research News | Disease/Infection News
Tags: Antibodies, Antibody, Assay, Children, Coronavirus, Coronavirus Disease COVID-19, Diagnostic, Enzyme, Healthcare, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article
