T-cells are critical for developing initial immunity towards pathogens such as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Memory T-cells could be reserved through adulthood to confer disease progression in the long term. The T-cell clonal response to SARS-CoV-2 vaccination is poorly understood in immunologically impaired individuals, as are the effects of risk factors of the vaccine response.
In a recent study published on the medRxiv* preprint server, a team of researchers investigate the post-vaccination clonal T-cell response and its relationship to antibody responses in inflammatory bowel disease (IBD) patients on anti-tumor necrosis factor (TNF) therapy.
Study: The T-cell clonal response to SARS-CoV-2 vaccination in inflammatory bowel disease patients is augmented by anti-TNF therapy and often deficient in antibody-responders. Image Credit: lmilian / Shutterstock.com
SARS-CoV-2 messenger ribonucleic acid (mRNA) vaccines are highly effective in preventing severe coronavirus disease 2019 (COVID-19). Vaccine-induced antibodies dominate protection against initial SARS-CoV-2 infection, whereas T-cells are largely responsible for controlling disease progression. Although patients with IBD on immune-modifying therapies produced post-vaccination antibodies, they were knocked down by anti-TNF therapy.
About the study
In the current study, the researchers collected samples of 303 SARS-CoV-2 vaccinated IBD patients, 53% of which were females, at four-time points including at their first dose, second dose, two weeks after receiving their second dose, and eight weeks after receiving their second dose. Of the 303 study participants, 53% received BNT262b (Pfizer), 42% mRNA 1273 (Moderna) and 5% Ad26CoV2 (Johnson & Johnson) vaccines.
The authors performed T-cell clonal analysis through T-cell receptor (TCR) immunosequencing and calculated the breadth and depth of the T-cell clonal response using reference datasets and compared them to antibody responses. The breadth and depth of T-cell response estimate the number of unique sequences and relative abundance of all the unique sequences to a given protein, respectively. Investigation of IBD patients permitted assessment of T-cell responses under selective and discrete modes of therapeutic immunosuppression.
The spike (S)-specific clonal response peaked two weeks after the completion of the vaccine regimen. No changes were seen for non-spike clones, thereby suggesting vaccine specificity.
Study findings
At the time of the first vaccine dose, the S-specific clonal response was low and reached a peak after two weeks of completion of the vaccine regimen, thus showing a three- and five-fold increase for T-cell clonal breadth and depth, respectively. From this peak, these levels again declined at eight weeks after the second dose of the vaccine but were still significantly elevated as compared to the timing of the first dose. The unchanged T-cell clonal metrics for non-spike clones suggest the specificity of the vaccine responses.
Spike-specific T-cell responses and antibody responses were significant but only moderately correlated at two weeks after the second dose of vaccine, which corresponds to the peak of both antibody and T-cell vaccine responses. Among patients who showed low antibody responses, T-cell clonal breadth and depth were low, thereby suggesting that an impaired humoral vaccine response also results in an impaired cellular response. However, in the majority of individuals who showed a lowered T-cell response, antibody levels were moderate or high.
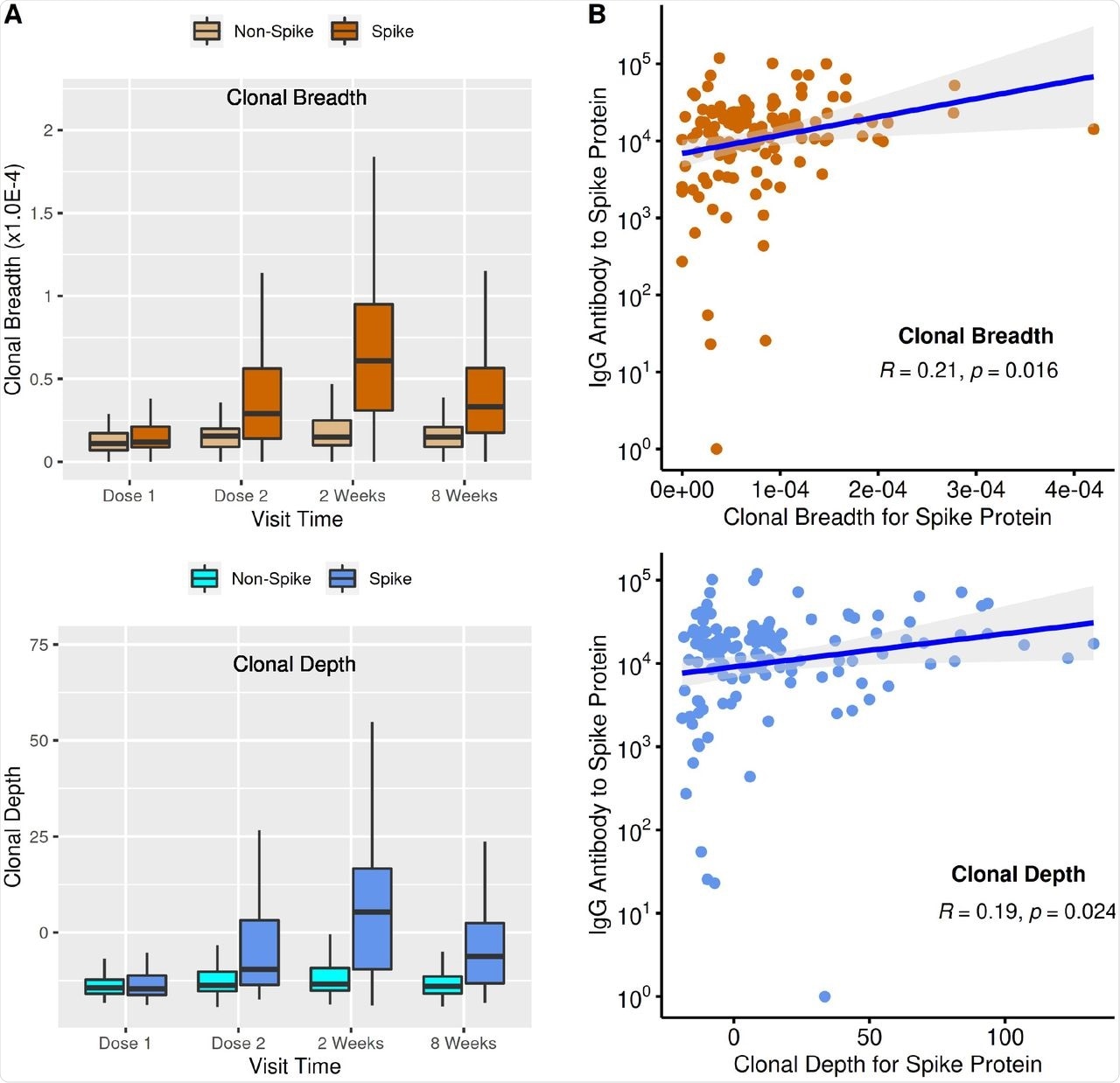 T-cell clonal response and antibody levels to SARS-CoV-2 immunization. (A) T-cell clonal response to SARS-CoV-2 vaccination. Box plots show mean, quartiles, and data range. Relative to dose 1, p values (mixed-effect model analysis with adjustment for age and sex) for dose 2, 2 weeks post 2nd vaccination, and 8 weeks post 2nd vaccination were: breadth (1.04E-8, 4.64E-25,1.08E-11); depth (9.87E-11, 2.42E-25,5.30E-14). (B) Comparison of T-cell clonal response metrics to anti-spike IgG levels (Spearman’s Correlation).
T-cell clonal response and antibody levels to SARS-CoV-2 immunization. (A) T-cell clonal response to SARS-CoV-2 vaccination. Box plots show mean, quartiles, and data range. Relative to dose 1, p values (mixed-effect model analysis with adjustment for age and sex) for dose 2, 2 weeks post 2nd vaccination, and 8 weeks post 2nd vaccination were: breadth (1.04E-8, 4.64E-25,1.08E-11); depth (9.87E-11, 2.42E-25,5.30E-14). (B) Comparison of T-cell clonal response metrics to anti-spike IgG levels (Spearman’s Correlation).
The S-specific clonal breadth was consistent across all age groups; however, the clonal depth reduced substantially with age. At two weeks after the second dose, there was no association between the gender and S-specific clonal responses. However, at eight weeks after the second dose, the T-cell clonal response increased in females as compared to males.
Disease type of IBD patients had minimal effects on the levels of S-specific clonal response to vaccines, as revealed by comparing statistics of Crohn’s disease and ulcerative colitis patients. Suppressive immunotherapy selectively but significantly affected the T-cell clonal depth.
The effects of steroids or molecular treatments on T-cell responses were insignificant. Study participants on anti-TNF monotherapy showed an augmented T-cell clonal depth after adjustment for age and sex.
The T-cell clonal responses to the two mRNA vaccines at two weeks after the second dose showed no significant differences but showed a marginal difference at eight weeks for clonal breadth favoring the mRNA-1273 vaccine. On the other hand, as compared to mRNA vaccination, Ad26.COV2.S induced a smaller S-specific clonal response after two and eight weeks of the second dose.
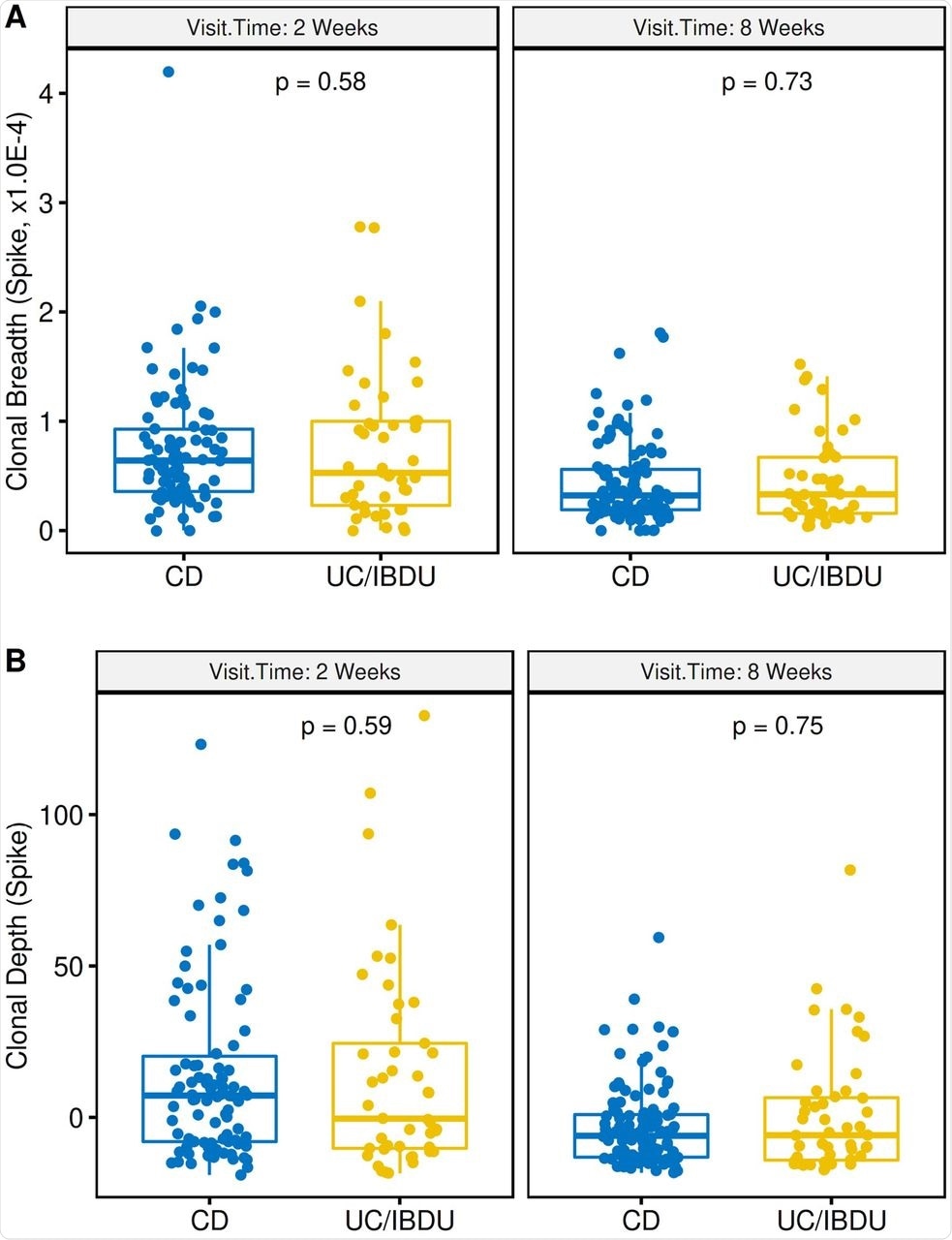 Effect of IBD disease type on spike T cell clonal response to vaccination. (A) T cell clonal breadth at week 2 (left) or week 8 (right) post second vaccination. (B) T cell clonal depth at week 2 (left) or week 8 (right) post second vaccination. CD, Crohn’s disease; UC/IC, Ulcerative colitis and indeterminant colitis. Box plots show mean, quartiles, and data range. P values are a mixed model analysis (with adjustment for age and gender) comparing dose 1 to either 2 weeks post 2nd vaccination (left) or 8 weeks (right) post 2nd vaccination.
Effect of IBD disease type on spike T cell clonal response to vaccination. (A) T cell clonal breadth at week 2 (left) or week 8 (right) post second vaccination. (B) T cell clonal depth at week 2 (left) or week 8 (right) post second vaccination. CD, Crohn’s disease; UC/IC, Ulcerative colitis and indeterminant colitis. Box plots show mean, quartiles, and data range. P values are a mixed model analysis (with adjustment for age and gender) comparing dose 1 to either 2 weeks post 2nd vaccination (left) or 8 weeks (right) post 2nd vaccination.
Conclusions
The current study accurately assesses the T-cell clonal responses to three SARS-CoV-2 vaccines to directly estimate SARS-CoV-2 S-specific T-cell clonal diversity (breadth) and clone size (depth) in immune-impaired individuals. The results revealed that T-cell responses were low in many of the study participants despite high antibody levels, thus indicating that age, sex, and certain immunotherapies are associated with the T-cell clonal response to SARS-CoV-2 vaccines.
These findings and observed differences in T-cell clonal responses due to vaccine type may help devise a reimmunization vaccine strategy for immune-impaired populations. In addition, studying the effects of anti-TNF therapy on vaccine responses would also prove beneficial.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Li, D., Xu, A., Mengesha, E., et al. (2021). The T-cell clonal response to SARS-CoV-2 vaccination in inflammatory bowel disease patients is augmented by anti-TNF therapy and often deficient in antibody-responders. medRxiv. doi:10.1101/2021.12.08.21267444. https://www.medrxiv.org/content/10.1101/2021.12.08.21267444v1.
Posted in: Medical Research News | Medical Condition News | Disease/Infection News
Tags: Antibodies, Antibody, Cell, Coronavirus, Crohn’s Disease, immunity, Immunization, Immunosuppression, Immunotherapy, Inflammatory Bowel Disease, Necrosis, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Tumor, Tumor Necrosis Factor, Ulcerative Colitis, Vaccine

Written by
Neha Mathur
Neha Mathur has a Master’s degree in Biotechnology and extensive experience in digital marketing. She is passionate about reading and music. When she is not working, Neha likes to cook and travel.
Source: Read Full Article
