Neurodegenerative diseases such as Alzheimer’s and Parkinson’s can be devastating to patients and their families. These diseases are difficult to diagnose before symptoms show, meaning it’s often already too late to reverse the damage to the central nervous system.
Early detection is key for management of symptoms and attempts to stall progression of the disease, but current knowledge is limited when it comes to tools that aid in early detection. That knowledge gap is being addressed through cutting-edge research by a team at The University of New Mexico led by Professor Eva Chi of the Department of Biomedical Engineering.
In order to understand complex diseases of the brain, one has to understand the complexity of human biology and the brain itself. Of particular importance is proteins—molecular structures inside a cell that can number into the tens-of-thousands—and their ability to dictate how cells function. Proteins start off with the same basic building blocks, called amino acids. The amino acids organize into a chain, and the unique function of the protein depends on how the amino acids are ordered in the chain. Once the amino acid ordering is complete, the protein chains fold themselves in various ways in order to bind to other molecules to perform certain tasks.
All proteins are made of the same building blocks; the folding of the protein into distinct shapes dictates its unique purpose inside the body. For example, digestive enzyme proteins break down our food into nutrients, and transport proteins such as hemoglobin carry substances throughout our body. Of particular interest to those who study brain degeneration are tau proteins inside neurons (brain cells) that help with cellular and nerve communication in the brain.
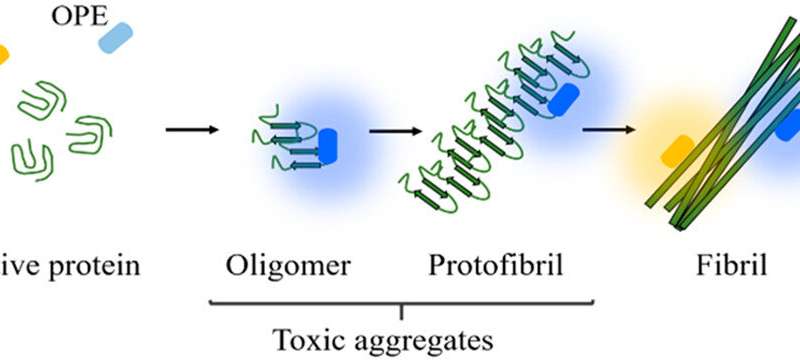
Protein folding is an intricate process, and as such, a lot can go wrong inside the cell. Protein folds can fail altogether, or an error in the protein chain could cause a misfold. Some of these misfolds have been linked by research scientists to numerous diseases in humans, especially when the misfolded proteins stick together. The resulting sticky clumps of proteins are called protein aggregates.
“Proteins have such important functions in the body, and once they do something else such as aggregate, it can have devasting consequences in the body, creating the potential for systemic and neurodegenerative diseases,” says Chi.
Previous research over the past decade has shown a link between degenerative brain diseases and aggregation of tau proteins inside neurons (tauopathy), as well as plaque-forming clumps of protein fragments called amyloid beta that disrupt the pathways between the cells. Scientists hypothesize that these protein aggregates form in the brain long before symptoms appear, and Chi’s research is focused on detection of these aggregates using a type of biosensor. Through past research, Chi and her team have developed a highly responsive biosensor called Oligo(p-phenylene ethynylene) electrolytes, or OPEs. OPEs are as a molecular structure created in a lab that can regulate electrical signals between neurons, as well as light up under a microscope when interacting with certain types of proteins.
Aggregates form inside one cell at the start, and as a disease such as Alzheimer’s progresses into the next stages, the aggregates recruit more healthy proteins inside the cell before spreading to multiple cells in the brain. Since Alzheimer’s, Parkinson’s, and similar diseases are not infectious, is unclear how the aggregation spreads from cell to cell. Mice models can track functionality through cognitive tests, but researchers cannot yet track biochemical changes inside a living human brain. Chi hopes the OPE sensors will also shine some light on this process.
“These diseases have a stage based on what the brain looks like, and the disease spreads throughout the brain, but we don’t know how it spreads. With other types of problems in the body, there are tests—X-rays, MRIs—but there is nothing for aggregates in the brain, and it’s something the field has been working towards,” says Chi. “The goal is to discover the next generation of sensors that can detect the protein aggregates that are more relevant to causing these diseases. In the long run, these sensors, if effective, will work along the lines of brain imaging that can detect the size, location, and cell-to-cell spread of the aggregates.”
Using mouse models, rat models, and donated human brain tissue in her lab, Chi takes proteins from these models in test tubes and treats them chemically to form aggregates. Her OPE sensors are added, and once the sensors find the aggregates, they bond to them and light up. Chi and her students then look at the results under a powerful microscope to see the features of the proteins and their sensors.
“Fundamental interactions between the sensor and the aggregate is the main focus,” Chi explains. “The sensor can seek out and find these aggregates and could potentially work to repair the damage. This knowledge can be applied for other purposes, such as sensors for antimicrobial applications, or used as therapies.”
Source: Read Full Article
