EXCLUSIVE: MDMA ‘will be rolled out in hospitals US by 2024’: Researchers will submit trial data in MONTHS and start FDA approval process — after rave drug ‘cured’ two-thirds of PTSD patients in study
- Final trials have been completed, with an FDA application due mid next year
- MDMA could be approved for therapeutic use just six months after that
- Over two thirds no longer met the threshold for a PTSD diagnosis in clinical trials
MDMA could be available in US hospitals in 2024 after showing promise as a powerful treatment for PTSD.
Researchers behind a landmark, federally-funded trial told DailyMail.com they expect to submit a new drug application within months.
The US Food and Drug Administration will make a decision on approval possibly as soon as six months later.
The move could see MDMA offered to some of the 12 million American adults who suffer with post traumatic stress disorder (PTSD).
Also known as ecstasy or molly, the drug is popular in rave culture, where it is used to dance all night long and feel more connected to the music and other ravers.
But it has become part of a new frontier of psychedelics that are being repurposed as drugs for trauma and depression, along with ketamine, magic mushrooms and LSD.
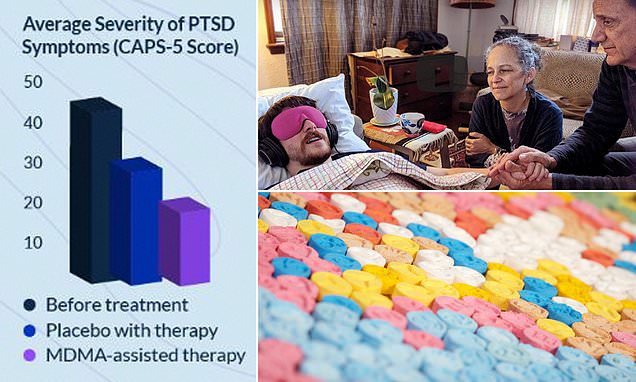
A trial of 90 patients with severe PTSD in 2021 showed that 88 per cent went into remission after taking the drug accompanied by therapy.
The second phase 3 trial of MDMA therapy for PTSD in the US was completed this month, testing the drug on patients whose PTSD is not as severe, as well as on more people of color. Data from the trial is yet to be released.
The Multidisciplinary Association for Psychedelic Studies (MAPS) — a nonprofit organization raising awareness of psychedelics — is running the trials.

Patients take a standard dose of the drug while under supervision. Sessions with a therapist then help people to come to terms with their trauma

Clinical trial participants were given 80 mg or 120 mg of MDMA — the standard amount for a pill — plus a supplementary dose of 40-60mg
A spokesperson told DailyMail.com: ‘We anticipate filing our NDA in the third quarter of 2023 [July to September].
‘Based on that estimated filing date we anticipate a possible approval in the second quarter of 2024 [April to June] and launch in the fourth quarter of 2024 [October to December].’
The latest study was completed ahead of schedule, meaning a New Drug Application could be submitted to the FDA by mid-2023.
The FDA’s review teams could take just six months to approve the drug, meaning it could be approved as early as April 2024 and rolled out in the autumn.
The drug is likely to be given in a clinic, similar to how the trials were conducted.
Participants were given 80 mg or 120 mg of MDMA — which is roughly the amount in the average ecstasy pill sold on the street.
Patients wore headphones and an eye mask, with a researchers who sat with them for eight hours to oversee their reaction. They were then given a booster dose of 40-60mg about two hours into the session.
Ecstasy, known chemically as MDMA or molly, has been used by clubbers for decades due to its effects in helping keep people awake.
It can come in the form of various pills and often takes about 30 minutes for its long-lasting effects to kick in, which can include feelings of love.
In the US, the jail term can be as severe as 40 years in some states.
In the UK, possession of any form of ecstasy – considered a Class A drug – comes with a potential jail term of up to seven years.
Drug campaigners warn the biggest risk of taking MDMA revolves around the fact that many users are unaware of what is in the substance they are taking.
It can include other drugs, such as PMA, which can be fatal in lower doses than MDMA itself.
The next morning, they had a 90-minute session with a therapist who helped them talk about, and process, their experience.
Results showed 67 per cent of participants no longer qualified for a PTSD diagnosis after just three MDMA therapy sessions. In total, 88 per cent of people had significant improvements in their symptoms.
Current treatments for PTSD include harsh antidepressants which suppress the immune system and cause a range of side effects, as well as some talking therapies.
While these drugs can help, they are not very effective in patients with severe PTSD, and the results fade over time.
MDA is thought to rewire connections in the brain, dampening the part that makes people scared, allowing them to open up with a therapist and face their trauma head on, instead of burying it.
The psychedelic also quietens the amygdala — the fear center in the brain — so that people can rationalize their trauma and comprehend that they survived it.
Some 90 patients took part in the first phase 3 trial, and were randomly assigned to receive MDMA assisted therapy or a placebo, along with 12 therapy sessions.
A letter in May from the US Department of Health and Human Services reinforced the FDA’s ‘anticipated approval’ of psychedelic therapies ‘within approximately 24 months’.
PTSD can occur after a distressing event, and can cause flashbacks, nightmares and intense anxiety.
The disorder affects 12 million adults in the US every year, and roughly six per cent of the population will have it at some point in their life.
A study in March this year by the University of California found that giving MDMA to people with PTSD doubled their chance of getting better through counselling.
Two thirds of PTSD sufferers given a 40mg dose of the party drug before therapy no longer suffered with the condition after two months.
By contrast, one in three of the control group who received standard counselling alone were cured over the same period of time.
The MDMA combination even worked on patients with the most severe PTSD and those with drug and alcohol abuse problems, experts said.
Researchers said the ‘feelings of trust and closeness’ caused by the drug helped them open up to psychiatrists and make better progress.
If MDMA therapy is rolled out, it could open the floodgates for more psychedelics pitched for approval.
Psilocybin, LSD and ayahuasca are all being tested in clinical trials.
Source: Read Full Article