In mice with high-risk neuroblastoma, tumors disappeared in response to a new combination treatment with precision medicines, a recent study from University of Gothenburg researchers shows. This is a vital step toward a potentially curative treatment for a form of cancer affecting young children that is currently difficult to treat.
The study, published in the journal Nature Communications, is the result of collaboration between researchers from the Universities of Gothenburg, Sweden and Ghent, Belgium.
Neuroblastoma is a form of childhood cancer that affects the peripheral nervous system (PNS)—that is, the system excluding the brain and spinal cord. In Sweden, 20–30 children are diagnosed annually. The cancer may start in the adrenal glands, for instance, but tumors can occur throughout the body.
“In some cases, the disease can heal and disappear by itself, but aggressive forms of neuroblastoma have a more unfavorable prognosis. The present treatment regimes are very hard for children to undergo and side effects can have consequences for the rest of their lives,” says Ruth Palmer, professor of molecular cell biology at the University of Gothenburg, who leads one of the research groups behind the new study.
Tumors disappeared in mice
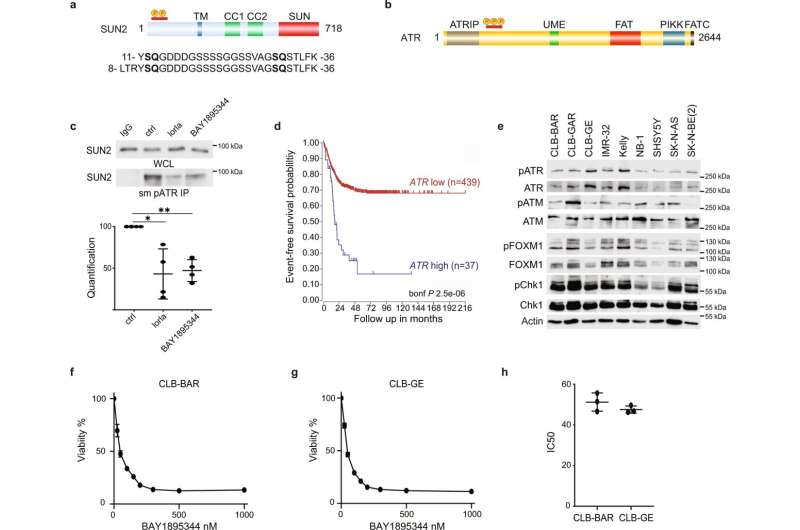
The study shows that a treatment combining two different precision medicines, an ATR inhibitor and an ALK inhibitor, eliminates neuroblastoma growth in mice.
“After 14 days’ treatment, the tumors had completely vanished in two independent mouse models. One of the mice relapsed after 200 days, which is a very long time for a mouse, but all the other mice are still alive,” says Dan Emil Lind, researcher in the group and one of the publication’s first authors.
“We’re surprised by the very positive result. It’s remarkable that a two-week protocol that combines these two precision medicines can lead to complete tumor regression in two separate mouse models of neuroblastoma,” says the other first author, Joanna Szydzik, a Ph.D. student in the group when the work was done.
Follow-up underway
“In experiments in culture with human cell lines, we see the same RNA and protein expression patterns as we do in mice treated with this new regime, showing we’re on the right track,” says Jimmy Van den Eynden of the Cancer Research Institute at Ghent University, who led the Belgian part of the work.
To date, the research group does not precisely understand why this combination treatment works so well. However, an important part of their hypothesis is that the mouse immune system received signals from the tumor cells that caused the natural immune cells to infiltrate and destroy the tumor cells.
“Our results are beyond our expectations. We’ve now started a follow-up with other ATR inhibitors to investigate how they affect the mouse immune system. We want to understand why this combination treatment with precision medicines against ATR and ALK is so good,” Palmer says.
Imbalanced expression
The molecular mechanisms whereby neuroblastoma arise are only partly known. What probably occurs is that defects appear in the development of the PNS, resulting in an imbalance that favors formation of neuroblastoma. The underlying defects in the cell may be that an important gene has been lost, that there is overexpression and/or activation of a specific protein, or that a particular protein or gene has mutated.
A quarter of all high-risk neuroblastomas arise when the oncogene MYCN is overexpressed. In many cases, this overexpression occurs in combination with an increase in the activity of the ALK protein. When such oncogenes are expressed and active in a cancer cell, it tends to divide faster, and “replication stress” can then occur.
More options needed
The results from this study suggest that patients with neuroblastoma, and especially those in high-risk groups where the combination of the MYCN and ALK genes lead to replication stress, may benefit from drug treatment with ATR inhibitors.
Source: Read Full Article
