The arrival of the Omicron (B.1.1.529) variant has brought into question the effectiveness of current COVID-19 treatments in preventing infection and death. New research published in the preprint bioRxiv* server suggests the efficacy of most monoclonal antibody treatments are ineffective against Omicron.
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 275 million people and caused over 5.3 million deaths worldwide. The SARS-CoV-2 virus has evolved on several occasions, leading to several variants of concerns with spike protein mutations that give it a better chance at evading the host’s immune system.
The latest variant of concern, Omicron, has more than double the mutations than Delta and has been the cause behind a surge of infections in both unvaccinated and vaccinated individuals. Since its discovery in late November, Omicron has been reported in many countries worldwide, including the United Kingdom and the United States.
Monoclonal antibody treatments from Regeneron, Lilly, and Celltrion completely lost neutralizing activity when faced with Omicron. However, monoclonal antibody treatments offered by AstraZeneca and Vir Biotechnology retained partial activity.
The study authors suggest more research identifying monoclonal antibodies that target the most highly conserved residues on the SARS-CoV-2 spike protein is necessary for adequate protection against Omicron and other future variants with mutated spike proteins.
 Study: An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies. Image Credit: Andrii Vodolazhskyi / Shutterstock
Study: An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies. Image Credit: Andrii Vodolazhskyi / Shutterstock
How they did it
The research team collected a sample of Omicron from an infected individual living in the United States. Then, they replicated the virus in cells expressing transmembrane protease serine 2 to prevent any additional mutations near the furin cleavage site in the spike protein.
The Omicron model resembled the strain first identified in South Africa, incorporating most of Omicron’s spike protein mutations. The only exception was the R346K mutation as it has been reported in approximately 8% of strains.
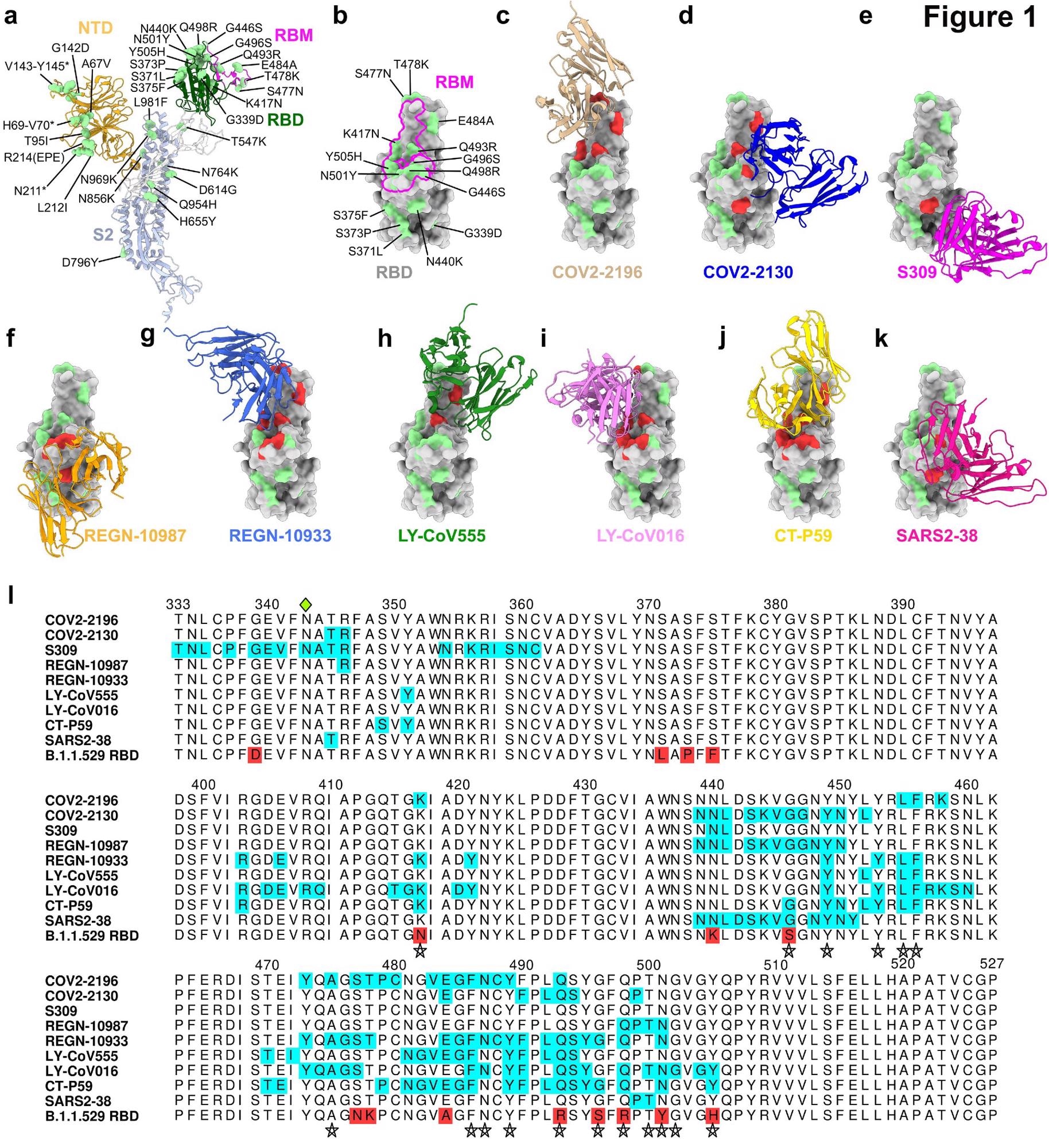
Omicron causes a loss of inhibitory activity in monoclonal antibodies
Because there are numerous mutations on Omicron’s spike protein, the researchers first investigated how mutations on the receptor-binding motif affected the binding of monoclonal antibodies.
The monoclonal antibodies tested in the study were those that had emergency use authorization or were in advanced clinical trials.
Every monoclonal antibody had structurally defined recognition sites changed in the Omicron spiked protein. However, the level of recognition differed between antibodies.
Monoclonal antibody treatments —REGN10933, REGN10987, LY-CoV555, LV-CoV016, CT-P59 and SARS2-38 —had no effect against Omicron. There continued to be an absence of neutralizing activity when researchers tested antibodies at the highest concentration.
Other monoclonal antibodies showed a partial reduction in inhibitory activity. COV2-2130 and COV2-2196 had an approximate 12- to 150-fold decrease in neutralizing power against Omicron. In contrast, S309 showed a 2-fold reduction in neutralization.
Monoclonal antibody cocktails in current medical use — REGN10933/REGN10987 and LY112 CoV555/LV-CoV016 — had no effect against Omicron. Another cocktail, COV2-
2130/COV2-2196, had about a 12-fold decrease in inhibitory activity.
Human ACE2 expression alters neutralization from monoclonal antibodies
Prior research suggests human ACE2 expression may influence the neutralizing activity of monoclonal antibodies. Additionally, Omicron spike protein mutations may increase binding interactions with human ACE2.
With this in mind, the researchers repeated the cellular study, but this time studied the potential role human ACE2 may play in decreasing neutralization from monoclonal antibodies.
Individual or monoclonal antibody cocktails successfully neutralized an earlier version of SARS-CoV-2. However, the REGN10933, REGN10987, LY-CoV555, LV-CoV016, SARS2-38, and CT-P59 monoclonal antibodies were completely ineffective against Omicron. Antibody cocktails in clinical use also lost their inhibitory activity.
COV2-2196 showed a 16-fold reduction and the COV2-2130/COV2-2196 combination treatment had an 11-fold reduction in neutralization.
In cells expressing human ACE2, the researchers encountered “unexpected” differences in inhibitory activity from COV2-2130 and S309 monoclonal antibodies. COV2-2130 showed no change in neutralizing activity from cells expressing or not expressing human ACE2. The S309 monoclonal antibody treatment showed only a 6-fold decrease in neutralizing activity against Omicron compared to an earlier SARS-CoV-2 strain.
The above results suggest monoclonal antibodies belonging to the AstraZeneca combination treatment or Vir Biotechnology still exert some inhibitory activity towards Omicron. However, the researchers note that more experiments looking into these treatments are needed to validate its clinical use against Omicron.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- VanBlargan LA, et al. (2021). An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies. bioRxiv. doi: https://doi.org/10.1101/2021.12.15.472828, https://www.biorxiv.org/content/10.1101/2021.12.15.472828v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: ACE2, Antibodies, Antibody, Biotechnology, Coronavirus, Coronavirus Disease COVID-19, CT, Efficacy, Immune System, Monoclonal Antibody, Mutation, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Serine, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Virus

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article
