The rapid spread of severe acute respiratory syndrome virus-2 (SARS-CoV-2) across the world has led to the rapid development of diagnostic kits that are highly sensitive and specific. Predominantly, these test kits are based on reverse transcriptase-polymerase chain reaction (RT-PCR) to detect total viral ribonucleic acid (RNA). One limitation of the RT-PCR test is that the relationship between a positive RT-PCR and viral infectivity is not prominent as the infection advances with time.
Prior studies have shown that SARS-CoV-2 remains in the upper respiratory tract for around 14.5 days after the onset of disease symptoms. However, many studies have also reported the presence of the viral RNA for several weeks, i.e., long past when most people are infectious. Owing to this reason, the Centers for Disease Control and Prevention (CDC) has developed new guidelines regarding isolation precautions.
According to the previous guideline, based on studies of viral infectivity in clinical samples, the isolation period for mild to moderately infected persons was ten days from the onset of symptoms. In the case of severely infected patients or immunocompromising conditions, isolation for a maximum of twenty days had been recommended. However, the current studies reveal some immunosuppressed patients may remain contagious for weeks, regardless of symptoms. This observation has increased the difficulty in framing strategies for isolation based on disease symptoms.
The lack of a rapid and high-throughput testing system to separate a diseased person from a healthy person makes it difficult to stop the transmission of infection. The available methods to determine infectivity are time-consuming and not practical. Thereby, scientists are interested in discovering molecular markers that could be correlated with infectivity. In this pretext, they have studied total RNA (positive and negative strands) of SARS-CoV-2 and subgenomic RNA (nucleocapsid (N), spike (S), envelope (E), and membrane (M), and six accessory proteins). The subgenomic RNA can be distinguished from the total RNA by the placement of alternate PCR primers. Evaluation of subgenomic RNA kinetics during infection revealed an absence of subgenomic RNA in the throat of SARS-CoV-2 patients up to 5 days after the onset of symptoms. Very little information is available about subgenomic RNA kinetics in hospitalized patients who have severe COVID-19 infection and in immunosuppressed patients.
A new paper, published on the medRxiv* preprint server, investigates the role of subgenomic RNA transcripts in SARS-CoV-2 infection. Earlier, scientists believed that subgenomic RNA could be traceable infective markers to monitor infectivity. In the current study, RT-PCR was used to amplify the total and subgenomic nucleoprotein gene (N) and envelope gene (E). Nasopharyngeal samples were collected from 190 hospitalized patients in Michigan between March 13, 2020, and June 10, 2020.
Researchers observed a strong correlation between the total RNA and subgenomic RNA. They found that the time points of post symptom onset are identical, and the decline in the infection rate is also the same for both total RNA and subgenomic RNA. The predictability of subgenomic copy numbers from total copy numbers indicates that recognition of subgenomic RNA does not add additional information about infectivity over and above that obtained from total copy number alone. Thereby, scientists conclude that no additional benefit would be incurred in evaluating subgenomic RNA.
In this research, scientists found that a negative subgenomic E RT-PCR positively correlates with time when patients are not infectious. Therefore, subgenomic E is not a valid marker to predict the infectivity of the virus. This study also reveals that the median number of days from the onset of symptoms to a negative subgenomic N RT-PCR is 25 days. Therefore, the use of subgenomic N as a marker for infectivity would significantly prolong isolation and not predict infectivity. This difference is likely due to the higher levels of expression of subgenomic N relative to subgenomic E.
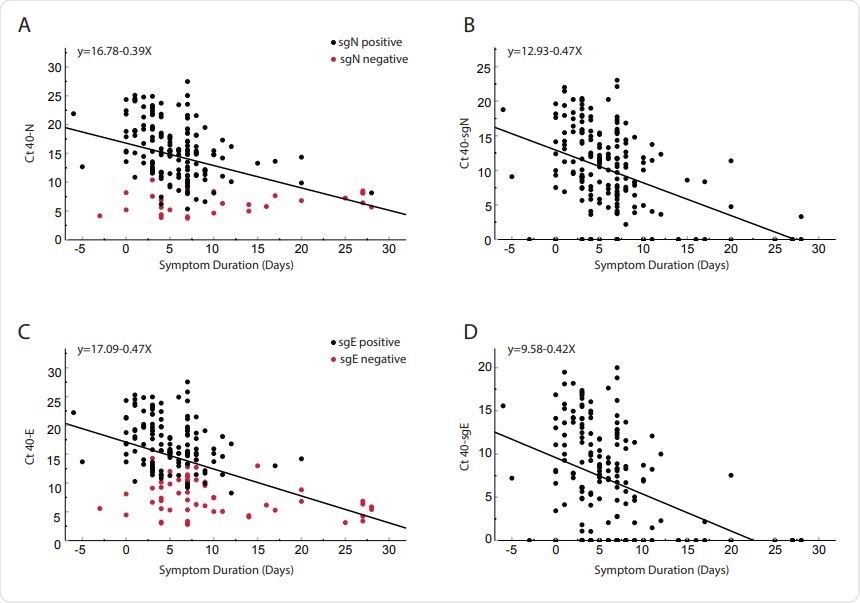
Previous research indicated that subgenomic RNA is a suitable marker for active infection because it degrades more rapidly than total RNA without replication. This result did not align with the results obtained in the current study. This difference in result is not due to differential degradation of subgenomic transcripts and total transcripts but rather owing to differential detection of transcripts.
The research concludes that the determination of infectivity by the evaluation of subgenomic RNA is not recommended. Similarly, regarding non-immunosuppressed patients, the copy number threshold of total RNA provides the same information that can be gained from the copy number of total RNA. One limitation of the current study is that the scientists have not isolated the virus from hospital samples and correlated it with total or subgenomic copy number. Only correlation of transcripts to symptom duration has been used as the deciding factor for infectivity.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- SARS-CoV-2 Total and Subgenomic RNA Viral Load in Hospitalized Patients, Derek E. Dimcheff, Andrew L. Valesano, Kalee E. Rumfelt, William J. Fitzsimmons, Christopher Blair, Carmen Mirabelli, Joshua G. Petrie, Emily T. Martin, Chandan Bhambhani, Muneesh Tewari, Adam S. Lauring medRxiv 2021.02.25.21252493; doi: https://doi.org/10.1101/2021.02.25.21252493, https://www.medrxiv.org/content/10.1101/2021.02.25.21252493v1
Posted in: Medical Research News | Disease/Infection News
Tags: Cell, Cell Culture, Coronavirus Disease COVID-19, Diagnostic, Electron, Gene, Hospital, Micrograph, Polymerase, Polymerase Chain Reaction, Research, Respiratory, Reverse Transcriptase, Ribonucleic Acid, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Throat, Virus

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article
